Symmetrical dimethylarginine methylation is required for the localization of SMN in Cajal bodies and pre-mRNA splicing
- PMID: 12486110
- PMCID: PMC2173973
- DOI: 10.1083/jcb.200207028
Symmetrical dimethylarginine methylation is required for the localization of SMN in Cajal bodies and pre-mRNA splicing
Abstract
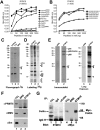
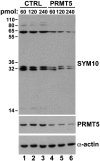
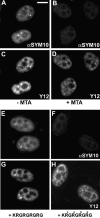
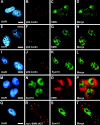
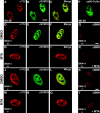
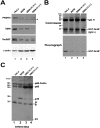
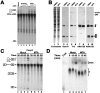
The nuclear structures that contain symmetrical dimethylated arginine (sDMA)-modified proteins and the role of this posttranslational modification is unknown. Here we report that the Cajal body is a major epitope in HeLa cells for an sDMA-specific antibody and that coilin is an sDMA-containing protein as analyzed by using the sDMA-specific antibody and matrix-assisted laser desorption ionization time of flight mass spectrometry. The methylation inhibitor 5'-deoxy-5'-methylthioadenosine reduces the levels of coilin methylation and causes the appearance of SMN-positive gems. In cells devoid of Cajal bodies, such as primary fibroblasts, sDMA-containing proteins concentrated in speckles. Cells from a patient with spinal muscular atrophy, containing low levels of the methyl-binding protein SMN, localized sDMA-containing proteins in the nucleoplasm as a discrete granular pattern. Splicing reactions are efficiently inhibited by using the sDMA-specific antibody or by using hypomethylated nuclear extracts, showing that active spliceosomes contain sDMA polypeptides and suggesting that arginine methylation is important for efficient pre-mRNA splicing. Our findings support a model in which arginine methylation is important for the localization of coilin and SMN in Cajal bodies.
Figures
Similar articles
-
The relationship between SMN, the spinal muscular atrophy protein, and nuclear coiled bodies in differentiated tissues and cultured cells.Exp Cell Res. 2000 May 1;256(2):365-74. doi: 10.1006/excr.2000.4858. Exp Cell Res. 2000. PMID: 10772809
-
Distinct domains of the spinal muscular atrophy protein SMN are required for targeting to Cajal bodies in mammalian cells.J Cell Sci. 2006 Feb 15;119(Pt 4):680-92. doi: 10.1242/jcs.02782. Epub 2006 Jan 31. J Cell Sci. 2006. PMID: 16449324
-
Cajal body proteins SMN and Coilin show differential dynamic behaviour in vivo.J Cell Sci. 2003 May 15;116(Pt 10):2039-50. doi: 10.1242/jcs.00400. Epub 2003 Apr 1. J Cell Sci. 2003. PMID: 12679382
-
The Cajal body.Biochim Biophys Acta. 2008 Nov;1783(11):2108-15. doi: 10.1016/j.bbamcr.2008.07.016. Epub 2008 Aug 3. Biochim Biophys Acta. 2008. PMID: 18755223 Review.
-
Cajal bodies and coilin--moving towards function.J Cell Biol. 2002 Oct 14;159(1):17-21. doi: 10.1083/jcb.200206111. Epub 2002 Oct 14. J Cell Biol. 2002. PMID: 12379800 Free PMC article. Review.
Cited by
-
In vitro RNase and nucleic acid binding activities implicate coilin in U snRNA processing.PLoS One. 2012;7(4):e36300. doi: 10.1371/journal.pone.0036300. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558428 Free PMC article.
-
Protein arginine methyltransferase 5 is essential for growth of lung cancer cells.Biochem J. 2012 Sep 1;446(2):235-41. doi: 10.1042/BJ20120768. Biochem J. 2012. PMID: 22708516 Free PMC article.
-
Arginine methylation of vasa protein is conserved across phyla.J Biol Chem. 2010 Mar 12;285(11):8148-54. doi: 10.1074/jbc.M109.089821. Epub 2010 Jan 15. J Biol Chem. 2010. PMID: 20080973 Free PMC article.
-
Arginine methylation mediated by the Arabidopsis homolog of PRMT5 is essential for proper pre-mRNA splicing.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):19114-9. doi: 10.1073/pnas.1009669107. Epub 2010 Oct 18. Proc Natl Acad Sci U S A. 2010. PMID: 20956294 Free PMC article.
-
Thrombospondin-1 is a transcriptional repression target of PRMT6.J Biol Chem. 2009 Aug 7;284(32):21338-46. doi: 10.1074/jbc.M109.005322. Epub 2009 Jun 9. J Biol Chem. 2009. PMID: 19509293 Free PMC article.
References
-
- Baldwin, G.S., and P.R. Carnegie. 1971. Specific enzymic methylation of an arginine in the experimental allergic encephalomyelitis protein from human myelin. Science. 171:579–581. - PubMed
-
- Bedford, M.T., A. Frankel, M.B. Yaffe, S. Clarke, P. Leder, and S. Richard. 2000. Arginine methylation inhibits the binding of proline-rich ligands to Src homology 3, but not WW, domains. J. Biol. Chem. 275:16030–16036. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

