Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice
- PMID: 12481038
- PMCID: PMC139283
- DOI: 10.1073/pnas.222657399
Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice
Abstract
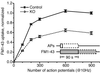
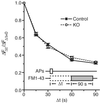
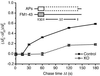
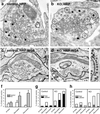
Synaptojanin 1 is a polyphosphoinositide phosphatase implicated in synaptic vesicle recycling. We used FM1-43 imaging and electron microscopy in cultured cortical neurons from control and synaptojanin 1 knockout mice to study how the absence of this protein affects specific steps of the synaptic vesicle cycle. Exoendocytosis after a moderate stimulus was unchanged. However, during prolonged stimulation, the regeneration of fusion-competent synaptic vesicles was severely impaired. In stimulated nerve terminals, there was a persistent accumulation of clathrin-coated vesicles and a backup of newly reformed vesicles in the cytomatrix-rich area around the synaptic vesicle cluster. These findings demonstrate that synaptojanin 1 function is needed for the progression of recycling vesicles to the functional synaptic vesicle pool.
Figures
Similar articles
-
Phosphorylation of Synaptojanin Differentially Regulates Endocytosis of Functionally Distinct Synaptic Vesicle Pools.J Neurosci. 2016 Aug 24;36(34):8882-94. doi: 10.1523/JNEUROSCI.1470-16.2016. J Neurosci. 2016. PMID: 27559170 Free PMC article.
-
Synaptojanin 2 functions at an early step of clathrin-mediated endocytosis.Curr Biol. 2003 Apr 15;13(8):659-63. doi: 10.1016/s0960-9822(03)00241-0. Curr Biol. 2003. PMID: 12699622
-
Synaptojanin and Endophilin Mediate Neck Formation during Ultrafast Endocytosis.Neuron. 2018 Jun 27;98(6):1184-1197.e6. doi: 10.1016/j.neuron.2018.06.005. Neuron. 2018. PMID: 29953872 Free PMC article.
-
Endophilin and synaptojanin hook up to promote synaptic vesicle endocytosis.Neuron. 2003 Nov 13;40(4):665-7. doi: 10.1016/s0896-6273(03)00726-8. Neuron. 2003. PMID: 14622570 Review.
-
Synaptic vesicle endocytosis.Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a005645. doi: 10.1101/cshperspect.a005645. Cold Spring Harb Perspect Biol. 2012. PMID: 22763746 Free PMC article. Review.
Cited by
-
Trisomy for synaptojanin1 in Down syndrome is functionally linked to the enlargement of early endosomes.Hum Mol Genet. 2012 Jul 15;21(14):3156-72. doi: 10.1093/hmg/dds142. Epub 2012 Apr 17. Hum Mol Genet. 2012. PMID: 22511594 Free PMC article.
-
The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis.Mol Cell Biol. 2005 Apr;25(8):2910-23. doi: 10.1128/MCB.25.8.2910-2923.2005. Mol Cell Biol. 2005. PMID: 15798181 Free PMC article.
-
The C2H2 zinc-finger protein SYD-9 is a putative posttranscriptional regulator for synaptic transmission.Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10450-10455. doi: 10.1073/pnas.0602073103. Epub 2006 Jun 27. Proc Natl Acad Sci U S A. 2006. PMID: 16803962 Free PMC article.
-
The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring.J Neurosci. 2004 Oct 6;24(40):8641-50. doi: 10.1523/JNEUROSCI.2892-04.2004. J Neurosci. 2004. PMID: 15470129 Free PMC article.
-
Live imaging of synaptic vesicle release and retrieval in dopaminergic neurons.Front Neural Circuits. 2009 Jun 1;3:3. doi: 10.3389/neuro.04.003.2009. eCollection 2009. Front Neural Circuits. 2009. PMID: 19521540 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

