The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells
- PMID: 12477810
- PMCID: PMC140579
- DOI: 10.1128/jvi.77.1.57-67.2003
The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells
Abstract
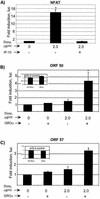
Kaposi's sarcoma-associated herpesvirus (KSHV/human herpesvirus 8 [HHV-8]) is a gamma-2-herpesvirus responsible for Kaposi's sarcoma as well as primary effusion lymphoma (PEL). KSHV is a lymphotropic virus that has pirated many mammalian genes involved in inflammation, cell cycle control, and angiogenesis. Among these is the early lytic viral G protein-coupled receptor (vGPCR), a homologue of the human interleukin-8 (IL-8) receptor. When expressed, vGPCR is constitutively active and can signal via mitogen- and stress-activated kinases. In certain models it activates the transcriptional potential of NF-kappaB and activator protein 1 (AP-1) and induces vascular endothelial growth factor (VEGF) production. Despite its importance to the pathogenesis of all KSHV-mediated disease, little is known about vGPCR activity in hematopoietic cells. To study the signaling potential and downstream effects of vGPCR in such cells, we have developed PEL cell lines that express vGPCR under the control of an inducible promoter. The sequences required for tetracycline-mediated induction were cloned into a plasmid containing adeno-associated virus type 2 elements to enhance integration efficiency. This novel plasmid permitted studies of vGPCR activity in naturally infected KSHV-positive lymphocytes. We show that vGPCR activates ERK-2 and p38 in PEL cells. In addition, it increases the transcription of reporter genes under the control of AP-1, NF-kappaB, CREB, and NFAT, a Ca(2+)-dependent transcription factor important to KSHV lytic gene expression. vGPCR also increases the transcription of KSHV open reading frames 50 and 57, thereby displaying broad potential to affect viral transcription patterns. Finally, vGPCR signaling results in increased PEL cell elaboration of KSHV vIL-6 and VEGF, two growth factors involved in KSHV-mediated disease pathogenesis.
Figures




Similar articles
-
The KSHV G protein-coupled receptor signals via multiple pathways to induce transcription factor activation in primary effusion lymphoma cells.Oncogene. 2004 Jan 15;23(2):514-23. doi: 10.1038/sj.onc.1207021. Oncogene. 2004. PMID: 14724579
-
The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha.Cancer Res. 2000 Sep 1;60(17):4873-80. Cancer Res. 2000. PMID: 10987301
-
G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator.Nature. 1998 Jan 1;391(6662):86-9. doi: 10.1038/34193. Nature. 1998. PMID: 9422510
-
Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies.Transl Res. 2013 Aug;162(2):77-92. doi: 10.1016/j.trsl.2013.03.004. Epub 2013 Apr 6. Transl Res. 2013. PMID: 23567332 Free PMC article. Review.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
Cited by
-
Molecular mechanisms deployed by virally encoded G protein-coupled receptors in human diseases.Annu Rev Pharmacol Toxicol. 2013;53:331-54. doi: 10.1146/annurev-pharmtox-010510-100608. Epub 2012 Oct 22. Annu Rev Pharmacol Toxicol. 2013. PMID: 23092247 Free PMC article. Review.
-
Attenuation of p38 MAPK activity upon contact inhibition in fibroblasts.Mol Cell Biochem. 2008 Jan;308(1-2):65-73. doi: 10.1007/s11010-007-9613-4. Epub 2007 Sep 29. Mol Cell Biochem. 2008. PMID: 17906919
-
Regulation of growth signalling and cell cycle by Kaposi's sarcoma-associated herpesvirus genes.Int J Exp Pathol. 2004 Dec;85(6):305-19. doi: 10.1111/j.0959-9673.2004.00407.x. Int J Exp Pathol. 2004. PMID: 15566428 Free PMC article. Review.
-
Cell cycle regulation during viral infection.Methods Mol Biol. 2014;1170:165-227. doi: 10.1007/978-1-4939-0888-2_10. Methods Mol Biol. 2014. PMID: 24906315 Free PMC article. Review.
-
Hypoxia-inducible factor-1 alpha as a therapeutic target for primary effusion lymphoma.PLoS Pathog. 2017 Sep 18;13(9):e1006628. doi: 10.1371/journal.ppat.1006628. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28922425 Free PMC article.
References
-
- Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. - PubMed
-
- Aoki, Y., and G. Tosato. 1999. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood 94:4247-4254. - PubMed
-
- Aoki, Y., and G. Tosato. 2001. Vascular endothelial growth factor/vascular permeability factor in the pathogenesis of primary effusion lymphomas. Leuk. Lymphoma 41:229-237. - PubMed
-
- Arima, N., and C. Tei. 2001. HTLV-I Tax related dysfunction of cell cycle regulators and oncogenesis of adult T cell leukemia. Leuk. Lymphoma 40:267-278. - PubMed
-
- Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

