Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production
- PMID: 12468729
- PMCID: PMC151204
- DOI: 10.1105/tpc.007906
Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production
Abstract
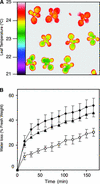
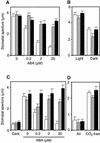
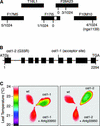
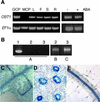
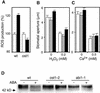
During drought, the plant hormone abscisic acid (ABA) triggers stomatal closure, thus reducing water loss. Using infrared thermography, we isolated two allelic Arabidopsis mutants (ost1-1 and ost1-2) impaired in the ability to limit their transpiration upon drought. These recessive ost1 mutations disrupted ABA induction of stomatal closure as well as ABA inhibition of light-induced stomatal opening. By contrast, the ost1 mutations did not affect stomatal regulation by light or CO(2), suggesting that OST1 is involved specifically in ABA signaling. The OST1 gene was isolated by positional cloning and was found to be expressed in stomatal guard cells and vascular tissue. In-gel assays indicated that OST1 is an ABA-activated protein kinase related to the Vicia faba ABA-activated protein kinase (AAPK). Reactive oxygen species (ROS) were shown recently to be an essential intermediate in guard cell ABA signaling. ABA-induced ROS production was disrupted in ost1 guard cells, whereas applied H(2)O(2) or calcium elicited the same degree of stomatal closure in ost1 as in the wild type. These results suggest that OST1 acts in the interval between ABA perception and ROS production. The relative positions of ost1 and the other ABA-insensitive mutations in the ABA signaling network (abi1-1, abi2-1, and gca2) are discussed.
Figures


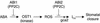
Similar articles
-
Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells.New Phytol. 2013 Dec;200(4):1049-63. doi: 10.1111/nph.12469. Epub 2013 Sep 3. New Phytol. 2013. PMID: 24033256
-
Involvement of OST1 Protein Kinase and PYR/PYL/RCAR Receptors in Methyl Jasmonate-Induced Stomatal Closure in Arabidopsis Guard Cells.Plant Cell Physiol. 2016 Aug;57(8):1779-90. doi: 10.1093/pcp/pcw102. Epub 2016 May 20. Plant Cell Physiol. 2016. PMID: 27354421
-
A link between magnesium-chelatase H subunit and sucrose nonfermenting 1 (SNF1)-related protein kinase SnRK2.6/OST1 in Arabidopsis guard cell signalling in response to abscisic acid.J Exp Bot. 2015 Oct;66(20):6355-69. doi: 10.1093/jxb/erv341. Epub 2015 Jul 13. J Exp Bot. 2015. PMID: 26175350 Free PMC article.
-
Signaling Transduction of ABA, ROS, and Ca2+ in Plant Stomatal Closure in Response to Drought.Int J Mol Sci. 2022 Nov 26;23(23):14824. doi: 10.3390/ijms232314824. Int J Mol Sci. 2022. PMID: 36499153 Free PMC article. Review.
-
Nitric oxide in guard cells as an important secondary messenger during stomatal closure.Front Plant Sci. 2013 Oct 29;4:425. doi: 10.3389/fpls.2013.00425. Front Plant Sci. 2013. PMID: 24194741 Free PMC article. Review.
Cited by
-
Compound stress response in stomatal closure: a mathematical model of ABA and ethylene interaction in guard cells.BMC Syst Biol. 2012 Nov 25;6:146. doi: 10.1186/1752-0509-6-146. BMC Syst Biol. 2012. PMID: 23176679 Free PMC article.
-
AHL-priming functions via oxylipin and salicylic acid.Front Plant Sci. 2015 Jan 14;5:784. doi: 10.3389/fpls.2014.00784. eCollection 2014. Front Plant Sci. 2015. PMID: 25642235 Free PMC article. Review.
-
Structural basis and functions of abscisic acid receptors PYLs.Front Plant Sci. 2015 Feb 19;6:88. doi: 10.3389/fpls.2015.00088. eCollection 2015. Front Plant Sci. 2015. PMID: 25745428 Free PMC article. Review.
-
The Fundamental Role of NOX Family Proteins in Plant Immunity and Their Regulation.Int J Mol Sci. 2016 May 27;17(6):805. doi: 10.3390/ijms17060805. Int J Mol Sci. 2016. PMID: 27240354 Free PMC article. Review.
-
PYR/PYL/RCAR abscisic acid receptors regulate K+ and Cl- channels through reactive oxygen species-mediated activation of Ca2+ channels at the plasma membrane of intact Arabidopsis guard cells.Plant Physiol. 2013 Oct;163(2):566-77. doi: 10.1104/pp.113.219758. Epub 2013 Jul 30. Plant Physiol. 2013. PMID: 23899646 Free PMC article.
References
-
- Assmann, S.M., and Wang, X.-Q. (2001). From milliseconds to millions of years: Guard cells and environmental responses. Curr. Opin. Plant Biol. 4, 421–428. - PubMed
-
- Blatt, M.R. (2000). Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 16, 221–241. - PubMed
-
- Blazquez, M.A., Soowal, L.N., Lee, I., and Weigel, D. (1997). LEAFY expression and flower initiation in Arabidopsis. Development 124, 3835–3844. - PubMed
-
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

