Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity
- PMID: 12451131
- PMCID: PMC6758728
- DOI: 10.1523/JNEUROSCI.22-23-10313.2002
Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity
Abstract
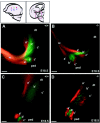
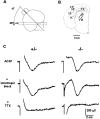
This study is concerned with the role of impulse activity and synaptic transmission in early thalamocortical development. Disruption of the gene encoding SNAP-25, a component of the soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor complex required for regulated neuroexocytosis, eliminates evoked but not spontaneous neurotransmitter release (Washbourne et al., 2002). The Snap25 null mutant mouse provides an opportunity to test whether synaptic activity is required for prenatal neural development. We found that evoked release is not needed for at least the gross formation of the embryonic forebrain, because the major features of the diencephalon and telencephalon were normal in the null mutant mouse. However, half of the homozygous mutants showed undulation of the cortical plate, which in the most severely affected brains was accompanied by a marked reduction of calbindin-immunoreactive neurons. Carbocyanine dye tracing of the thalamocortical fiber pathway revealed normal growth kinetics and fasciculation patterns between embryonic days 17.5 and 19. As in normal mice, mutant thalamocortical axons reach the cortex, accumulate below the cortical plate, and then start to extend side-branches in the subplate and deep cortical plate. Multiple carbocyanine dye placements in the cortical convexity revealed normal overall topography of both early thalamocortical and corticofugal projections. Electrophysiological recordings from thalamocortical slices confirmed that thalamic axons were capable of conducting action potentials to the cortex. Thus, our data suggest that axonal growth and early topographic arrangement of these fiber pathways do not rely on activity-dependent mechanisms requiring evoked neurotransmitter release. Intercellular communication mediated by constitutive secretion of transmitters or growth factors, however, might play a part.
Figures


Similar articles
-
Termination and initial branch formation of SNAP-25-deficient thalamocortical fibres in heterochronic organotypic co-cultures.Eur J Neurosci. 2012 May;35(10):1586-94. doi: 10.1111/j.1460-9568.2012.08120.x. Eur J Neurosci. 2012. PMID: 22607004 Free PMC article.
-
Choreography of early thalamocortical development.Cereb Cortex. 2003 Jun;13(6):661-9. doi: 10.1093/cercor/13.6.661. Cereb Cortex. 2003. PMID: 12764042
-
Defects in reciprocal projections between the thalamus and cerebral cortex in the early development of Fezl-deficient mice.J Comp Neurol. 2007 Jul 20;503(3):454-65. doi: 10.1002/cne.21401. J Comp Neurol. 2007. PMID: 17503485
-
Thalamocortical synaptic connections: efficacy, modulation, inhibition and plasticity.Rev Neurosci. 2001;12(2):159-73. doi: 10.1515/revneuro.2001.12.2.159. Rev Neurosci. 2001. PMID: 11392456 Review.
-
Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain.Eur J Neurosci. 2012 May;35(10):1573-85. doi: 10.1111/j.1460-9568.2012.08119.x. Eur J Neurosci. 2012. PMID: 22607003 Free PMC article. Review.
Cited by
-
Mental development is associated with cortical connectivity of the ventral and nonspecific thalamus of preterm newborns.Brain Behav. 2020 Oct;10(10):e01786. doi: 10.1002/brb3.1786. Epub 2020 Aug 13. Brain Behav. 2020. PMID: 32790242 Free PMC article.
-
Interaction between environmental and genetic factors modulates schizophrenic endophenotypes in the Snap-25 mouse mutant blind-drunk.Hum Mol Genet. 2009 Dec 1;18(23):4576-89. doi: 10.1093/hmg/ddp425. Epub 2009 Sep 3. Hum Mol Genet. 2009. PMID: 19729413 Free PMC article.
-
Methodological Approach for Optogenetic Manipulation of Neonatal Neuronal Networks.Front Cell Neurosci. 2017 Aug 14;11:239. doi: 10.3389/fncel.2017.00239. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28848399 Free PMC article.
-
Maturation of Complex Synaptic Connections of Layer 5 Cortical Axons in the Posterior Thalamic Nucleus Requires SNAP25.Cereb Cortex. 2021 Mar 31;31(5):2625-2638. doi: 10.1093/cercor/bhaa379. Cereb Cortex. 2021. PMID: 33367517 Free PMC article.
-
Differential effect on myelination through abolition of activity-dependent synaptic vesicle release or reduction of overall electrical activity of selected cortical projections in the mouse.J Anat. 2019 Sep;235(3):452-467. doi: 10.1111/joa.12974. Epub 2019 Mar 22. J Anat. 2019. PMID: 30901089 Free PMC article.
References
-
- Bagri A, Martin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. - PubMed
-
- Behar TN, Smith SV, Kennedy RT, McKenzie JM, Maric I, Barker JL. GABA(B) receptors mediate motility signals for migrating embryonic cortical cells. Cereb Cortex. 2001;11:744–753. - PubMed
-
- Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. - PubMed
-
- Catalano SM, Robertson RT, Killackey HP. Individual axon morphology and thalamocortical topography in developing rat somatosensory cortex. J Comp Neurol. 1996;367:36–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
