Vps51 is part of the yeast Vps fifty-three tethering complex essential for retrograde traffic from the early endosome and Cvt vesicle completion
- PMID: 12446664
- PMCID: PMC1705970
- DOI: 10.1074/jbc.M210436200
Vps51 is part of the yeast Vps fifty-three tethering complex essential for retrograde traffic from the early endosome and Cvt vesicle completion
Abstract
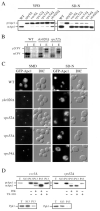
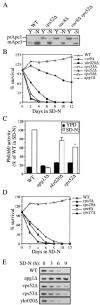
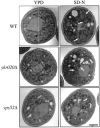
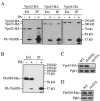
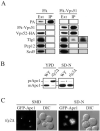
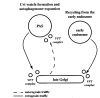
Autophagy, pexophagy, and the Cvt pathway are processes that deliver hydrolytic enzymes and substrates to the yeast vacuole/lysosome via double-membrane cytosolic vesicles. Whereas these pathways operate under different nutritional conditions, they all employ common machinery with only a few specific factors assisting in the choice of the delivery program and the membrane source for the sequestering vesicle. We found that the YKR020w gene product is essential for Cvt vesicle formation but not for pexophagy or induction of autophagy. Autophagosomes in the ykr020wdelta mutant, however, have a reduced size. We demonstrate that Ykr020 is a subunit of the Vps fifty-three tethering complex, composed of Vps52, Vps53, and Vps54, which is required for retrograde traffic from the early endosome back to the late Golgi, and for this reason we named it Vps51. This complex participates in a fusion event together with Tlg1 and Tlg2, two SNAREs also shown to be necessary for Cvt vesicle assembly. In particular, those factors are essential to correctly target the prApe1-Cvt19-Cvt9 complex to the preautophagosomal structure, the site of Cvt vesicle formation.
Figures


Similar articles
-
Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway.J Biol Chem. 2004 Jul 16;279(29):29889-94. doi: 10.1074/jbc.M404399200. Epub 2004 May 11. J Biol Chem. 2004. PMID: 15138258 Free PMC article.
-
Early stages of the secretory pathway, but not endosomes, are required for Cvt vesicle and autophagosome assembly in Saccharomyces cerevisiae.Mol Biol Cell. 2004 May;15(5):2189-204. doi: 10.1091/mbc.e03-07-0479. Epub 2004 Mar 5. Mol Biol Cell. 2004. PMID: 15004240 Free PMC article.
-
Yeast dynamin associates with the GARP tethering complex for endosome-to-Golgi traffic.Eur J Cell Biol. 2017 Sep;96(6):612-621. doi: 10.1016/j.ejcb.2017.04.004. Epub 2017 May 8. Eur J Cell Biol. 2017. PMID: 28521960
-
Molecular machinery required for autophagy and the cytoplasm to vacuole targeting (Cvt) pathway in S. cerevisiae.Curr Opin Cell Biol. 2002 Aug;14(4):468-75. doi: 10.1016/s0955-0674(02)00343-5. Curr Opin Cell Biol. 2002. PMID: 12383798 Review.
-
Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae.Biochim Biophys Acta. 2005 Jul 10;1744(3):438-54. doi: 10.1016/j.bbamcr.2005.04.004. Biochim Biophys Acta. 2005. PMID: 15913810 Review.
Cited by
-
Loss of the homotypic fusion and vacuole protein sorting or golgi-associated retrograde protein vesicle tethering complexes results in gentamicin sensitivity in the yeast Saccharomyces cerevisiae.Antimicrob Agents Chemother. 2006 Feb;50(2):587-95. doi: 10.1128/AAC.50.2.587-595.2006. Antimicrob Agents Chemother. 2006. PMID: 16436714 Free PMC article.
-
Transport according to GARP: receiving retrograde cargo at the trans-Golgi network.Trends Cell Biol. 2011 Mar;21(3):159-67. doi: 10.1016/j.tcb.2010.11.003. Epub 2010 Dec 21. Trends Cell Biol. 2011. PMID: 21183348 Free PMC article. Review.
-
The P4-ATPase Drs2 interacts with and stabilizes the multisubunit tethering complex TRAPPIII in yeast.EMBO Rep. 2023 May 4;24(5):e56134. doi: 10.15252/embr.202256134. Epub 2023 Mar 16. EMBO Rep. 2023. PMID: 36929574 Free PMC article.
-
Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts.Autophagy. 2005 Jul;1(2):101-9. doi: 10.4161/auto.1.2.1840. Epub 2005 Jul 11. Autophagy. 2005. PMID: 16874040 Free PMC article.
-
Soi3p/Rav1p functions at the early endosome to regulate endocytic trafficking to the vacuole and localization of trans-Golgi network transmembrane proteins.Mol Biol Cell. 2004 Jul;15(7):3196-209. doi: 10.1091/mbc.e03-10-0755. Epub 2004 Apr 16. Mol Biol Cell. 2004. PMID: 15090613 Free PMC article.
References
-
- Harding TM, Hefner-Gravink A, Thumm M, Klionsky DJ. J. Biol. Chem. 1996;271:17621–17624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

