ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway
- PMID: 12438613
- PMCID: PMC136725
- DOI: 10.1128/jvi.76.24.12877-12889.2002
ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway
Abstract
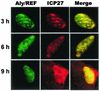
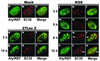
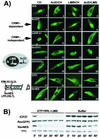
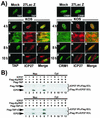
Herpes simplex virus type 1 (HSV-1) protein ICP27 facilitates the export of viral intronless mRNAs. ICP27 shuttles between the nucleus and cytoplasm, which has been shown to require a leucine-rich nuclear export sequence (NES). ICP27 export was reported to be sensitive to the CRM1 inhibitor leptomycin B (LMB) in HSV-1-infected cells but not in Xenopus oocytes, where ICP27 interacts with the export factor Aly/REF to access the TAP export pathway. Here, we show that ICP27 interacts with Aly/REF in HSV-1-infected mammalian cells and that Aly/REF stimulates export of viral intronless RNAs but does not cross-link to these RNAs. During infection, Aly/REF was no longer associated with splicing factor SC35 but moved into structures that colocalized with ICP27, suggesting that ICP27 recruits Aly/REF from spliceosomes to viral intronless RNAs. Further, ICP27 was found to interact in vivo with TAP but not with CRM1. In vitro export assays showed that ICP27 export was not sensitive to LMB but was blocked by a dominant-negative TAP deletion mutant lacking the nucleoporin interaction domain. These data suggest that ICP27 uses the TAP pathway to export viral RNAs. Interestingly, the leucine-rich N-terminal sequence was required for efficient export, even though ICP27 export was LMB insensitive. Thus, this region is required for efficient ICP27 export but does not function as a CRM1-dependent NES.
Figures



Similar articles
-
ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export.J Virol. 2005 Apr;79(7):3949-61. doi: 10.1128/JVI.79.7.3949-3961.2005. J Virol. 2005. PMID: 15767397 Free PMC article.
-
The cellular RNA export receptor TAP/NXF1 is required for ICP27-mediated export of herpes simplex virus 1 RNA, but the TREX complex adaptor protein Aly/REF appears to be dispensable.J Virol. 2009 Jul;83(13):6335-46. doi: 10.1128/JVI.00375-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369354 Free PMC article.
-
The interaction of the cellular export adaptor protein Aly/REF with ICP27 contributes to the efficiency of herpes simplex virus 1 mRNA export.J Virol. 2013 Jul;87(13):7210-7. doi: 10.1128/JVI.00738-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637401 Free PMC article.
-
The many roles of the regulatory protein ICP27 during herpes simplex virus infection.Front Biosci. 2008 May 1;13:5241-56. doi: 10.2741/3078. Front Biosci. 2008. PMID: 18508584 Review.
-
RNA export: insights from viral models.Essays Biochem. 2000;36:115-27. doi: 10.1042/bse0360115. Essays Biochem. 2000. PMID: 12471907 Review.
Cited by
-
Kaposi's sarcoma-associated herpesvirus ORF57 is not a bona fide export factor.J Virol. 2012 Dec;86(23):13089-94. doi: 10.1128/JVI.00606-12. Epub 2012 Sep 19. J Virol. 2012. PMID: 22993146 Free PMC article.
-
KSHV ORF57, a protein of many faces.Viruses. 2015 Feb 10;7(2):604-33. doi: 10.3390/v7020604. Viruses. 2015. PMID: 25674768 Free PMC article. Review.
-
Deciphering novel host-herpesvirus interactions by virion proteomics.Front Microbiol. 2012 May 28;3:181. doi: 10.3389/fmicb.2012.00181. eCollection 2012. Front Microbiol. 2012. PMID: 22783234 Free PMC article.
-
ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation.EMBO J. 2003 Apr 1;22(7):1608-19. doi: 10.1093/emboj/cdg166. EMBO J. 2003. PMID: 12660167 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus ORF57 protein enhances mRNA accumulation independently of effects on nuclear RNA export.J Virol. 2007 Sep;81(18):9990-8. doi: 10.1128/JVI.00896-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609285 Free PMC article.
References
-
- Bachi, A., I. C. Braun, J. P. Rodrigues, N. Pante, K. Ribbeck, C. von Kobbe, U. Kutay, M. Wilm, D. Gorlich, M. Carmo-Fonseca, and E. Izaurralde. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6:136-158. - PMC - PubMed
-
- Braun, I. C., A. Herold, M. Rode, E. Conti, and E. Izaurralde. 2001. Overexpression of TAP/p15 heterodimers bypasses nuclear retention and stimulates nuclear mRNA export. J. Biol. Chem. 276:20536-20543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

