Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells
- PMID: 12438417
- PMCID: PMC2173094
- DOI: 10.1083/jcb.200208154
Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells
Abstract
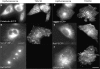
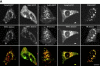
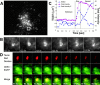
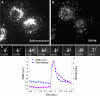
Similar to its role in secretory cells, calcium triggers exocytosis in nonsecretory cells. This calcium-dependent exocytosis is essential for repair of membrane ruptures. Using total internal reflection fluorescence microscopy, we observed that many organelles implicated in this process, including ER, post-Golgi vesicles, late endosomes, early endosomes, and lysosomes, were within 100 nm of the plasma membrane (in the evanescent field). However, an increase in cytosolic calcium led to exocytosis of only the lysosomes. The lysosomes that fused were predominantly predocked at the plasma membrane, indicating that calcium is primarily responsible for fusion and not recruitment of lysosomes to the cell surface.
Figures


Similar articles
-
Differential properties of GTP- and Ca(2+)-stimulated exocytosis from large dense core vesicles.Traffic. 2006 Apr;7(4):416-28. doi: 10.1111/j.1600-0854.2006.00394.x. Traffic. 2006. PMID: 16536740
-
Visualization of regulated exocytosis with a granule-membrane probe using total internal reflection microscopy.Mol Biol Cell. 2004 Oct;15(10):4658-68. doi: 10.1091/mbc.e04-02-0149. Epub 2004 Jul 28. Mol Biol Cell. 2004. PMID: 15282339 Free PMC article.
-
Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells.J Cell Biol. 1997 Apr 7;137(1):93-104. doi: 10.1083/jcb.137.1.93. J Cell Biol. 1997. PMID: 9105039 Free PMC article.
-
Lysosomes and plasma membrane repair.Curr Top Membr. 2019;84:1-16. doi: 10.1016/bs.ctm.2019.08.001. Epub 2019 Sep 3. Curr Top Membr. 2019. PMID: 31610859 Review.
-
There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII.Trends Cell Biol. 2005 Nov;15(11):626-31. doi: 10.1016/j.tcb.2005.09.001. Epub 2005 Sep 15. Trends Cell Biol. 2005. PMID: 16168654 Review.
Cited by
-
Evidence for lysosomal exocytosis and release of aggrecan-degrading hydrolases from hypertrophic chondrocytes, in vitro and in vivo.Biol Open. 2012 Apr 15;1(4):318-28. doi: 10.1242/bio.2012547. Epub 2012 Feb 10. Biol Open. 2012. PMID: 23213422 Free PMC article.
-
Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms.Traffic. 2010 Apr;11(4):533-47. doi: 10.1111/j.1600-0854.2009.01029.x. Epub 2009 Dec 17. Traffic. 2010. PMID: 20028487 Free PMC article.
-
Palmitoylation-dependent association with CD63 targets the Ca2+ sensor synaptotagmin VII to lysosomes.J Cell Biol. 2010 Nov 1;191(3):599-613. doi: 10.1083/jcb.201003021. J Cell Biol. 2010. PMID: 21041449 Free PMC article.
-
The secreted inhibitor of invasive cell growth CREG1 is negatively regulated by cathepsin proteases.Cell Mol Life Sci. 2021 Jan;78(2):733-755. doi: 10.1007/s00018-020-03528-5. Epub 2020 May 8. Cell Mol Life Sci. 2021. PMID: 32385587 Free PMC article.
-
Role of AP1 and Gadkin in the traffic of secretory endo-lysosomes.Mol Biol Cell. 2011 Jun 15;22(12):2068-82. doi: 10.1091/mbc.E11-03-0193. Epub 2011 Apr 27. Mol Biol Cell. 2011. PMID: 21525240 Free PMC article.
References
-
- Ayala, B.P., B. Vasquez, S. Clary, J.A. Tainer, K. Rodland, and M. So. 2001. The pilus-induced Ca2+ flux triggers lysosome exocytosis and increases the amount of Lamp1 accessible to Neisseria IgA1 protease. Cell Microbiol. 3:265–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

