Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts
- PMID: 12438412
- PMCID: PMC2173095
- DOI: 10.1083/jcb.200205098
Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts
Abstract
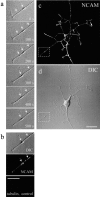
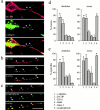
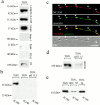
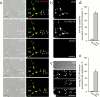
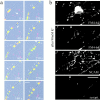
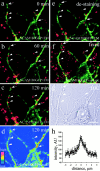
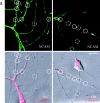
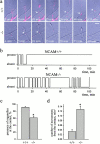
Transformation of a contact between axon and dendrite into a synapse is accompanied by accumulation of the synaptic machinery at this site, being delivered in intracellular organelles mainly of TGN origin. Here, we report that in cultured hippocampal neurons, TGN organelles are linked via spectrin to clusters of the neural cell adhesion molecule (NCAM) in the plasma membrane. These complexes are translocated along neurites and trapped at sites of initial neurite-to-neurite contacts within several minutes after initial contact formation. The accumulation of TGN organelles at contacts with NCAM-deficient neurons is reduced when compared with wild-type cells, suggesting that NCAM mediates the anchoring of intracellular organelles in nascent synapses.
Figures

Similar articles
-
NCAM induces CaMKIIalpha-mediated RPTPalpha phosphorylation to enhance its catalytic activity and neurite outgrowth.J Cell Biol. 2008 Sep 22;182(6):1185-200. doi: 10.1083/jcb.200803045. J Cell Biol. 2008. PMID: 18809727 Free PMC article.
-
Trans-Golgi network delivery of synaptic proteins in synaptogenesis.J Cell Sci. 2004 Jan 26;117(Pt 3):381-8. doi: 10.1242/jcs.00956. J Cell Sci. 2004. PMID: 14702384 Review.
-
Developmentally regulated masking of an intracellular epitope of the 180 kDa isoform of the neural cell adhesion molecule NCAM.J Neurosci Res. 1997 Jul 15;49(2):161-75. J Neurosci Res. 1997. PMID: 9272639
-
Binding of alphaII spectrin to 14-3-3beta is involved in NCAM-dependent neurite outgrowth.Mol Cell Neurosci. 2010 Sep;45(1):66-74. doi: 10.1016/j.mcn.2010.05.013. Epub 2010 Jun 18. Mol Cell Neurosci. 2010. PMID: 20598904
-
Polysialic acid-neural cell adhesion molecule in brain plasticity: from synapses to integration of new neurons.Brain Res Rev. 2007 Nov;56(1):101-18. doi: 10.1016/j.brainresrev.2007.05.014. Epub 2007 Jul 4. Brain Res Rev. 2007. PMID: 17658613 Review.
Cited by
-
Conditional ablation of the neural cell adhesion molecule reduces precision of spatial learning, long-term potentiation, and depression in the CA1 subfield of mouse hippocampus.J Neurosci. 2004 Feb 18;24(7):1565-77. doi: 10.1523/JNEUROSCI.3298-03.2004. J Neurosci. 2004. PMID: 14973228 Free PMC article.
-
Dynamic aspects of CNS synapse formation.Annu Rev Neurosci. 2007;30:425-50. doi: 10.1146/annurev.neuro.29.051605.112830. Annu Rev Neurosci. 2007. PMID: 17417940 Free PMC article. Review.
-
Spatial control of exocytosis.Curr Opin Cell Biol. 2003 Aug;15(4):430-7. doi: 10.1016/s0955-0674(03)00074-7. Curr Opin Cell Biol. 2003. PMID: 12892783 Free PMC article. Review.
-
NCAM induces CaMKIIalpha-mediated RPTPalpha phosphorylation to enhance its catalytic activity and neurite outgrowth.J Cell Biol. 2008 Sep 22;182(6):1185-200. doi: 10.1083/jcb.200803045. J Cell Biol. 2008. PMID: 18809727 Free PMC article.
-
The neural cell adhesion molecule promotes FGFR-dependent phosphorylation and membrane targeting of the exocyst complex to induce exocytosis in growth cones.J Neurosci. 2011 Mar 9;31(10):3522-35. doi: 10.1523/JNEUROSCI.3109-10.2011. J Neurosci. 2011. PMID: 21389209 Free PMC article.
References
-
- Ahmari, S.E., J. Buchanan, and S.J. Smith. 2000. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3:445–451. - PubMed
-
- Burry, R.W. 1986. Presynaptic elements on artificial surfaces. A model for the study of development and regeneration of synapses. Neurochem. Pathol. 5:345–360. - PubMed
-
- Burry, R.W. 1991. Transitional elements with characteristics of both growth cones and presynaptic terminals observed in cell cultures of cerebellar neurons. J. Neurocytol. 20:124–132. - PubMed
-
- Cremer, H., R. Lange, A. Christoph, M. Plomann, G. Vopper, J. Roes, R. Brown, S. Baldwin, P. Kraemer, S. Scheff, et al. 1994. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 367:455–459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

