Localization, ion channel regulation, and genetic interactions during abscisic acid signaling of the nuclear mRNA cap-binding protein, ABH1
- PMID: 12427994
- PMCID: PMC166648
- DOI: 10.1104/pp.009480
Localization, ion channel regulation, and genetic interactions during abscisic acid signaling of the nuclear mRNA cap-binding protein, ABH1
Abstract
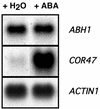
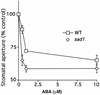
Abscisic acid (ABA) regulates developmental processes and abiotic stress responses in plants. We recently characterized a new Arabidopsis mutant, abh1, which shows ABA-hypersensitive regulation of seed germination, stomatal closing, and cytosolic calcium increases in guard cells (V. Hugouvieux, J.M. Kwak, J.I. Schroeder [2001] Cell 106: 477-487). ABH1 encodes the large subunit of a dimeric Arabidopsis mRNA cap-binding complex and in expression profiling experiments was shown to affect mRNA levels of a subset of genes. Here, we show that the dimeric ABH1 and AtCBP20 subunits are ubiquitously expressed. Whole-plant growth phenotypes of abh1 are described and properties of ABH1 in guard cells are further analyzed. Complemented abh1 lines expressing a green fluorescent protein-ABH1 fusion protein demonstrate that ABH1 mainly localizes in guard cell nuclei. Stomatal apertures were smaller in abh1 compared with wild type (WT) when plants were grown at 40% humidity, and similar at 95% humidity. Correlated with stomatal apertures from plants grown at 40% humidity, slow anion channel currents were enhanced and inward potassium channel currents were decreased in abh1 guard cells compared with WT. Gas exchange measurements showed similar primary humidity responses in abh1 and WT, which together with results from abh1/abi1-1 double-mutant analyses suggest that abh1 shows enhanced sensitivity to endogenous ABA. Double-mutant analyses of the ABA-hypersensitive signaling mutants, era1-2 and abh1, showed complex genetic interactions, suggesting that ABH1 and ERA1 do not modulate the same negative regulator in ABA signaling. Mutations in the RNA-binding protein sad1 showed hypersensitive ABA-induced stomatal closing, whereas hyl1 did not affect this response. These data provide evidence for the model that the mRNA-processing proteins ABH1 and SAD1 function as negative regulators in guard cell ABA signaling.
Figures






Similar articles
-
The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA.Plant Physiol. 2006 Jan;140(1):127-39. doi: 10.1104/pp.105.070318. Epub 2005 Dec 16. Plant Physiol. 2006. PMID: 16361522 Free PMC article.
-
An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis.Cell. 2001 Aug 24;106(4):477-87. doi: 10.1016/s0092-8674(01)00460-3. Cell. 2001. PMID: 11525733
-
The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production.Plant Physiol. 2007 Mar;143(3):1398-407. doi: 10.1104/pp.106.091298. Epub 2007 Jan 12. Plant Physiol. 2007. PMID: 17220365 Free PMC article.
-
mRNA cap binding proteins: effects on abscisic acid signal transduction, mRNA processing, and microarray analyses.Curr Top Microbiol Immunol. 2008;326:139-50. doi: 10.1007/978-3-540-76776-3_8. Curr Top Microbiol Immunol. 2008. PMID: 18630751 Review.
-
Signal transduction and ion channels in guard cells.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review.
Cited by
-
Open or close the gate - stomata action under the control of phytohormones in drought stress conditions.Front Plant Sci. 2013 May 13;4:138. doi: 10.3389/fpls.2013.00138. eCollection 2013. Front Plant Sci. 2013. PMID: 23717320 Free PMC article.
-
The cap-binding complex modulates ABA-responsive transcript splicing during germination in barley (Hordeum vulgare).Sci Rep. 2024 Aug 7;14(1):18278. doi: 10.1038/s41598-024-69373-9. Sci Rep. 2024. PMID: 39107424 Free PMC article.
-
Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana.Proc Natl Acad Sci U S A. 2008 Jun 24;105(25):8795-800. doi: 10.1073/pnas.0802493105. Epub 2008 Jun 12. Proc Natl Acad Sci U S A. 2008. PMID: 18550839 Free PMC article.
-
Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling.PLoS Biol. 2006 Oct;4(10):e312. doi: 10.1371/journal.pbio.0040312. PLoS Biol. 2006. PMID: 16968132 Free PMC article.
-
Alternative splicing in ABA signaling during seed germination.Front Plant Sci. 2023 Mar 16;14:1144990. doi: 10.3389/fpls.2023.1144990. eCollection 2023. Front Plant Sci. 2023. PMID: 37008485 Free PMC article. Review.
References
-
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. - PubMed
-
- Assmann SM, Snyder JA, Lee Y-RJ. ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ. 2000;23:387–395.
-
- Blatt MR, Armstrong F. Potassium channels of stomatal guard cells: abscisic acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta. 1993;191:330–341.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

