Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer's disease
- PMID: 12427828
- PMCID: PMC6757822
- DOI: 10.1523/JNEUROSCI.22-22-09733.2002
Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer's disease
Abstract
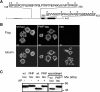
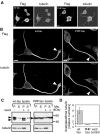
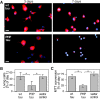
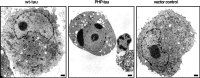
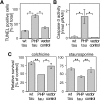
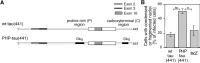
Aggregation and increased phosphorylation of tau at selected sites ("hyperphosphorylation") are histopathological hallmarks of Alzheimer's disease (AD). However, it is not known whether the tau pathology has a primary role during neuronal degeneration. To determine the role of tau hyperphosphorylation in AD, pseudohyperphosphorylated tau (PHP-tau) that simulates disease-like permanent, high stoichiometric tau phosphorylation and mimics structural and functional aspects of hyperphosphorylated tau was expressed in neural cells. In differentiated PC12 cells, PHP-tau exhibited reduced microtubule interaction and failed to stabilize the microtubule network compared with exogenously expressed wild-type tau (wt-tau). During longer culture, PHP-tau exerted a cytotoxic effect, whereas wt-tau was neutral. PHP-tau-mediated cytotoxicity was associated with an induction of apoptotic cell death as characterized by chromatin condensation, DNA fragmentation, and caspase-3 activation in the absence of detectable protein aggregates. Furthermore, PHP-tau expression specifically sensitized the cells for other apoptotic stimuli (colchicine and staurosporine). Herpes simplex virus-mediated overexpression of PHP-tau induced degeneration associated with an induction of apoptotic mechanisms also in terminally differentiated human CNS model neurons. Partially pseudophosphorylated constructs caused an intermediate toxicity. The data provide evidence for a neurotoxic "gain of function" of soluble tau during AD as a result of structural changes that are induced by a cumulative, high stoichiometric tau phosphorylation. PHP-tau-expressing cells and organisms could provide a useful system to identify mechanisms that contribute to tau-mediated toxicity.
Figures

Similar articles
-
Inverse and distinct modulation of tau-dependent neurodegeneration by presenilin 1 and amyloid-beta in cultured cortical neurons: evidence that tau phosphorylation is the limiting factor in amyloid-beta-induced cell death.J Neurochem. 2007 Jun;101(5):1303-15. doi: 10.1111/j.1471-4159.2006.04435.x. Epub 2007 Feb 7. J Neurochem. 2007. PMID: 17298384
-
Tau phosphorylation during apoptosis of human SH-SY5Y neuroblastoma cells.Brain Res. 2001 Dec 7;921(1-2):31-43. doi: 10.1016/s0006-8993(01)03074-8. Brain Res. 2001. PMID: 11720709
-
Tau and HMW tau phosphorylation and compartmentalization in apoptotic neuronal PC12 cells.J Neurosci Res. 2001 Oct 15;66(2):203-13. doi: 10.1002/jnr.1212. J Neurosci Res. 2001. PMID: 11592115
-
Tau pathology in Alzheimer disease and other tauopathies.Biochim Biophys Acta. 2005 Jan 3;1739(2-3):198-210. doi: 10.1016/j.bbadis.2004.09.008. Biochim Biophys Acta. 2005. PMID: 15615638 Review.
-
Role of microtubule-associated protein tau phosphorylation in Alzheimer's disease.J Huazhong Univ Sci Technolog Med Sci. 2017 Jun;37(3):307-312. doi: 10.1007/s11596-017-1732-x. Epub 2017 Jun 6. J Huazhong Univ Sci Technolog Med Sci. 2017. PMID: 28585125 Review.
Cited by
-
N-PEP-12--a novel peptide compound that protects cortical neurons in culture against different age and disease associated lesions.J Neural Transm (Vienna). 2005 Oct;112(10):1331-43. doi: 10.1007/s00702-005-0283-7. Epub 2005 Mar 7. J Neural Transm (Vienna). 2005. PMID: 15750682
-
The Evolution of Tau Phosphorylation and Interactions.Front Aging Neurosci. 2019 Sep 18;11:256. doi: 10.3389/fnagi.2019.00256. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31619983 Free PMC article.
-
The Crocus sativus Compounds trans-Crocin 4 and trans-Crocetin Modulate the Amyloidogenic Pathway and Tau Misprocessing in Alzheimer Disease Neuronal Cell Culture Models.Front Neurosci. 2019 Mar 26;13:249. doi: 10.3389/fnins.2019.00249. eCollection 2019. Front Neurosci. 2019. PMID: 30971876 Free PMC article.
-
The role of tau in neurodegeneration.Mol Neurodegener. 2009 Mar 11;4:13. doi: 10.1186/1750-1326-4-13. Mol Neurodegener. 2009. PMID: 19284597 Free PMC article.
-
Novel immunological approaches for the treatment of Alzheimer's disease.Zhongguo Xian Dai Shen Jing Ji Bing Za Zhi. 2014;14(3):139-151. doi: 10.3969/j.issn.1672-6731.2014.03.001. Zhongguo Xian Dai Shen Jing Ji Bing Za Zhi. 2014. PMID: 25429302 Free PMC article.
References
-
- Biernat J, Gustke N, Drewes G, Mandelkow E-M, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. - PubMed
-
- Braak E, Braak H, Mandelkow EM. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol Berl. 1994;87:554–567. - PubMed
-
- Brandt R, Lee G, Teplow DB, Shalloway D, Abdel-Ghany M. Differential effect of phosphorylation and substrate modulation on tau's ability to promote microtubule growth and nucleation. J Biol Chem. 1994;269:11776–11782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
