The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation
- PMID: 12419243
- PMCID: PMC3499980
- DOI: 10.1016/s0092-8674(02)01047-4
The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation
Abstract
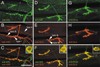
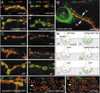
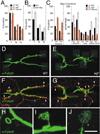
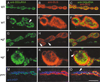
At vertebrate neuromuscular junctions (NMJs), Agrin plays pivotal roles in synapse development, but molecules that activate synapse formation at central synapses are largely unknown. Members of the Wnt family are well established as morphogens, yet recently they have also been implicated in synapse maturation. Here we demonstrate that the Drosophila Wnt, Wingless (Wg), is essential for synapse development. We show that Wg and its receptor are expressed at glutamatergic NMJs, and that Wg is secreted by synaptic boutons. Loss of Wg leads to dramatic reductions in target-dependent synapse formation, and new boutons either fail to develop active zones and postsynaptic specializations or these are strikingly aberrant. We suggest that Wg signals the coordinated development of pre- and postsynaptic compartments.
Figures

Similar articles
-
Notum coordinates synapse development via extracellular regulation of Wingless trans-synaptic signaling.Development. 2017 Oct 1;144(19):3499-3510. doi: 10.1242/dev.148130. Epub 2017 Aug 31. Development. 2017. PMID: 28860114 Free PMC article.
-
Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP.Proc Natl Acad Sci U S A. 2006 May 16;103(20):7841-6. doi: 10.1073/pnas.0600387103. Epub 2006 May 8. Proc Natl Acad Sci U S A. 2006. PMID: 16682643 Free PMC article.
-
Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors.BMC Cell Biol. 2003 May 2;4:4. doi: 10.1186/1471-2121-4-4. BMC Cell Biol. 2003. PMID: 12729465 Free PMC article.
-
Cellular mechanisms of wingless/Wnt signal transduction.Curr Top Dev Biol. 1999;43:153-90. doi: 10.1016/s0070-2153(08)60381-6. Curr Top Dev Biol. 1999. PMID: 9891886 Review.
-
Powerful Drosophila screens that paved the wingless pathway.Fly (Austin). 2014;8(4):218-25. doi: 10.4161/19336934.2014.985988. Epub 2015 Jan 20. Fly (Austin). 2014. PMID: 25565425 Free PMC article. Review.
Cited by
-
Autophagy promotes synapse development in Drosophila.J Cell Biol. 2009 Oct 5;187(1):71-9. doi: 10.1083/jcb.200907109. Epub 2009 Sep 28. J Cell Biol. 2009. PMID: 19786572 Free PMC article.
-
Secreted frizzled related protein 1 (Sfrp1) and Wnt signaling in innervated and denervated skeletal muscle.J Mol Histol. 2008 Jun;39(3):329-37. doi: 10.1007/s10735-008-9169-y. Epub 2008 Apr 5. J Mol Histol. 2008. PMID: 18392598
-
UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites.Nature. 2008 Oct 2;455(7213):669-73. doi: 10.1038/nature07291. Nature. 2008. PMID: 18776887 Free PMC article.
-
Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila.J Cell Biol. 2014 Mar 31;204(7):1157-72. doi: 10.1083/jcb.201308115. Epub 2014 Mar 24. J Cell Biol. 2014. PMID: 24662564 Free PMC article.
-
Postsynaptic assembly: a role for Wnt signaling.Dev Neurobiol. 2014 Aug;74(8):818-27. doi: 10.1002/dneu.22138. Epub 2013 Nov 15. Dev Neurobiol. 2014. PMID: 24105999 Free PMC article. Review.
References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. - PubMed
-
- Burden SJ. Wnts as retrograde signals for axon and growth cone differentiation. Cell. 2000;100:495–497. - PubMed
-
- Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the wingless morphogen gradient in the wing. Cell. 1998;93:767–777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

