Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2
- PMID: 12414536
- PMCID: PMC1850779
- DOI: 10.1016/S0002-9440(10)64466-5
Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2
Abstract
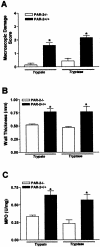
Proteinase-activated receptor (PAR)-2, a G-protein-coupled receptor for trypsin and mast cell tryptase, is highly expressed in the intestine. Luminal trypsin and tryptase are elevated in the colon of inflammatory bowel disease patients. We hypothesized that luminal proteinases activate PAR-2 and induce colonic inflammation. Mice received intracolonically PAR-2 agonists (trypsin, tryptase, and a selective PAR-2-activating peptide) or control drugs (boiled enzymes, inactive peptide) and inflammatory parameters were followed at various times after this treatment. Colonic administration of PAR-2 agonists up-regulated PAR-2 expression and induced an inflammatory reaction characterized by granulocyte infiltration, increased wall thickness, tissue damage, and elevated T-helper cell type 1 cytokine. The inflammation was maximal between 4 and 6 hours and was resolved 48 hours after the intracolonic administration. PAR-2 activation also increased paracellular permeability of the colon and induced bacterial trans-location into peritoneal organs. These proinflammatory and pathophysiological changes observed in wild-type mice were not detected in PAR-2-deficient mice. Luminal proteinases activate PAR-2 in the mouse colon to induce inflammation and disrupt the integrity of the intestinal barrier. Because trypsin and tryptase are found at high levels in the colon lumen of patients with Crohn's disease or ulcerative colitis, our data may bear directly on the pathophysiology of human inflammatory bowel diseases.
Figures





Similar articles
-
A role for proteinase-activated receptor-1 in inflammatory bowel diseases.J Clin Invest. 2004 Nov;114(10):1444-56. doi: 10.1172/JCI21689. J Clin Invest. 2004. Retraction in: J Clin Invest. 2006 Jul;116(7):2056. doi: 10.1172/jci21689r1. PMID: 15545995 Free PMC article. Retracted.
-
Activation of ion secretion via proteinase-activated receptor-2 in human colon.Am J Physiol Gastrointest Liver Physiol. 2002 Feb;282(2):G200-10. doi: 10.1152/ajpgi.00137.2001. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 11804840
-
Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats.Gastroenterology. 2002 Apr;122(4):1035-47. doi: 10.1053/gast.2002.32387. Gastroenterology. 2002. PMID: 11910355
-
Protease activated receptor 2: a new target for IBS treatment.Eur Rev Med Pharmacol Sci. 2008 Aug;12 Suppl 1:95-102. Eur Rev Med Pharmacol Sci. 2008. PMID: 18924448 Review.
-
Proteinase-activated receptors: a growing family of heptahelical receptors for thrombin, trypsin and tryptase.Biochem Soc Trans. 1999 Feb;27(2):246-54. doi: 10.1042/bst0270246. Biochem Soc Trans. 1999. PMID: 10093742 Review. No abstract available.
Cited by
-
Proteolytic bacteria expansion during colitis amplifies inflammation through cleavage of the external domain of PAR2.Gut Microbes. 2024 Jan-Dec;16(1):2387857. doi: 10.1080/19490976.2024.2387857. Epub 2024 Aug 22. Gut Microbes. 2024. PMID: 39171684 Free PMC article.
-
Rivaroxaban Induces Mucosal Healing in a Rat Model of Trinitrobenzene Sulfonic Acid-Induced Colitis.Med Princ Pract. 2015;24(5):470-6. doi: 10.1159/000431361. Epub 2015 Jun 20. Med Princ Pract. 2015. PMID: 26111863 Free PMC article.
-
Protease-activated receptor (PAR)2, but not PAR1, is involved in collateral formation and anti-inflammatory monocyte polarization in a mouse hind limb ischemia model.PLoS One. 2013 Apr 18;8(4):e61923. doi: 10.1371/journal.pone.0061923. Print 2013. PLoS One. 2013. PMID: 23637930 Free PMC article.
-
Increased intestinal permeability and gut dysbiosis in the R6/2 mouse model of Huntington's disease.Sci Rep. 2020 Oct 26;10(1):18270. doi: 10.1038/s41598-020-75229-9. Sci Rep. 2020. PMID: 33106549 Free PMC article.
-
Role of pancreatic trypsin in chronic esophagitis induced by gastroduodenal reflux in rats.J Gastroenterol. 2006 Mar;41(3):198-208. doi: 10.1007/s00535-005-1742-5. J Gastroenterol. 2006. PMID: 16699853
References
-
- Brass LF, Molino M: Protease-activated G protein-coupled receptors on human platelets and endothelial cells. Thromb Haemost 1997, 78:234-241 - PubMed
-
- Vergnolle N: Review article: proteinase-activated receptors-novel signals for gastrointestinal pathophysiology. Aliment Pharmacol Ther 2000, 14:257-266 - PubMed
-
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD: Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci 2001, 22:146-152 - PubMed
-
- Vu T, Hung D, Wheaton V, Coughlin S: Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64:1057-1068 - PubMed
-
- Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR: PAR3 is a cofactor for PAR4 activation by thrombin. Nature 2000, 404:609-613 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

