Tau assembly in inducible transfectants expressing wild-type or FTDP-17 tau
- PMID: 12414518
- PMCID: PMC1850799
- DOI: 10.1016/S0002-9440(10)64448-3
Tau assembly in inducible transfectants expressing wild-type or FTDP-17 tau
Abstract
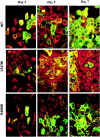
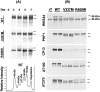
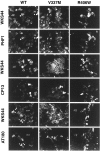
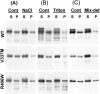
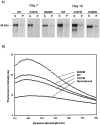
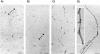
Conditional expression systems for 4-repeat wild-type (WT) tau or the corresponding mutants V337M and R406W were established in human neuroglioma H4 cells to study the effect of tau mutations on the physicochemical properties of tau, and to develop a cellular model for the formation of filamentous tau characteristic of frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) and Alzheimer's disease. Upon induction tau expression increased, reaching maximal levels at 5 to 7 days. WT tau was phosphorylated at amino acids T181, S202/T205, T231, and S396/S404. The R406W mutation decreased tau phosphorylation at each of these sites as did the V337M mutation except for S396/S404 sites that increased. Most tau in postnuclear cell lysates was recovered in the supernatant fraction after centrifugation at 200,000 x g. The amount of tau in the pellet fraction increased more in mutant transfectants compared to WT when the induction was extended beyond 5 days. This particulate tau could be partially extracted with salt, Triton X-100, or sarkosyl. Of the transfectants, R406W had the highest proportion of sarkosyl-insoluble tau by day 7. This insoluble fraction was thioflavin S-positive and contained 15- to 5-nm-wide filaments with tau immunoreactivities. The R406W filaments were more abundant than those detected in similar preparations from WT or V337M transfectants. At the light microscopy level, most tau was found with microtubules, or diffusely distributed in the cytoplasm, but none of this appeared thioflavin S-positive. The results suggest that conditional tau transfectants are in a pretangle stage making them an attractive model system for studying intracellular tangle accumulation and for testing potential therapeutic agents as inhibitors for tau aggregation.
Figures

Similar articles
-
Tau proteins with frontotemporal dementia-17 mutations have both altered expression levels and phosphorylation profiles in differentiated neuroblastoma cells.Neuroscience. 2001;108(4):701-12. doi: 10.1016/s0306-4522(01)00434-1. Neuroscience. 2001. PMID: 11738505
-
Missense point mutations of tau to segregate with FTDP-17 exhibit site-specific effects on microtubule structure in COS cells: a novel action of R406W mutation.J Neurosci Res. 2000 May 1;60(3):380-7. doi: 10.1002/(SICI)1097-4547(20000501)60:3<380::AID-JNR13>3.0.CO;2-5. J Neurosci Res. 2000. PMID: 10797541
-
Molecular analysis of mutant and wild-type tau deposited in the brain affected by the FTDP-17 R406W mutation.Am J Pathol. 2001 Feb;158(2):373-9. doi: 10.1016/S0002-9440(10)63979-X. Am J Pathol. 2001. PMID: 11159174 Free PMC article.
-
[Molecular analysis of tau deposited in the FTDP-17 brain].Rinsho Shinkeigaku. 2001 Dec;41(12):1107-10. Rinsho Shinkeigaku. 2001. PMID: 12235810 Review. Japanese.
-
Tau mutations in frontotemporal dementia FTDP-17 and their relevance for Alzheimer's disease.Biochim Biophys Acta. 2000 Jul 26;1502(1):110-21. doi: 10.1016/s0925-4439(00)00037-5. Biochim Biophys Acta. 2000. PMID: 10899436 Review.
Cited by
-
Pharmacological induction of heat shock proteins ameliorates toxicity of mutant PKCγ in spinocerebellar ataxia type 14.J Biol Chem. 2018 Sep 21;293(38):14758-14774. doi: 10.1074/jbc.RA118.002913. Epub 2018 Aug 9. J Biol Chem. 2018. PMID: 30093405 Free PMC article.
-
Two-dimensional electrophoresis of tau mutants reveals specific phosphorylation pattern likely linked to early tau conformational changes.PLoS One. 2009;4(3):e4843. doi: 10.1371/journal.pone.0004843. Epub 2009 Mar 17. PLoS One. 2009. PMID: 19290042 Free PMC article.
-
Isomerase Pin1 stimulates dephosphorylation of tau protein at cyclin-dependent kinase (Cdk5)-dependent Alzheimer phosphorylation sites.J Biol Chem. 2013 Mar 15;288(11):7968-7977. doi: 10.1074/jbc.M112.433326. Epub 2013 Jan 28. J Biol Chem. 2013. PMID: 23362255 Free PMC article.
-
The role of tau in neurodegenerative diseases and its potential as a therapeutic target.Scientifica (Cairo). 2012;2012:796024. doi: 10.6064/2012/796024. Epub 2012 Dec 19. Scientifica (Cairo). 2012. PMID: 24278740 Free PMC article. Review.
-
Increase in tau tyrosine phosphorylation correlates with the formation of tau aggregates.Brain Res Mol Brain Res. 2005 Aug 18;138(2):135-44. doi: 10.1016/j.molbrainres.2005.04.015. Brain Res Mol Brain Res. 2005. PMID: 15913839 Free PMC article.
References
-
- Spillantini MG, Van Swieten JC, Goedert M: Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Neurogenetics 2000, 2:193-205 - PubMed
-
- Heutink P: Untangling tau-related dementia. Hum Mol Genet 2000, 9:979-986 - PubMed
-
- Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Litchfield S, Smith A, Barnetson L, Smith AD: Relative roles of plaques and tangles in the dementia of Alzheimer’s disease: correlations using three sets of neuropathological criteria. Dementia 1995, 6:21-31 - PubMed
-
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hacket J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaf E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Kuei Che L, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd P, Hayward N, Kwok BJ, Schofield P, Andreadis A, Snowden J, Craufurd D, Neary D, Owen, Oostr B, Hardy J, Goate A, Van Swieten J, Mann D, Lynch T, Heutink P: Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393:702-705 - PubMed
-
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD: Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 1998, 43:815-825 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

