Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development
- PMID: 12397180
- PMCID: PMC137567
- DOI: 10.1073/pnas.222362099
Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development
Abstract
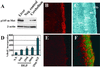
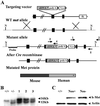
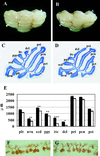
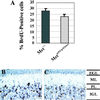
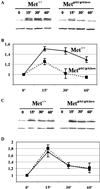
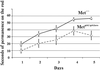
Cerebellar development occurs mainly postnatally and implies cell proliferation and migration. Hepatocyte growth factor (HGF) and Met are involved in mediating these responses in other tissues and are coexpressed in the cerebellum. Here we show that Met is localized in granule cell precursors and that cultures of these cells respond to HGF with proliferation. To study the role of HGF and Met in the cerebellum in vivo, we produced a viable hypomorphic Met mutant by knocking in the met locus a point mutation to abrogate the receptor Grb2-binding site. A similar mutant was previously described as perinatal lethal. In this "first-generation" knock-in the recombinant locus retained the Neo cassette (Met(grb2/grb2neo+)). In the knock-in presented here Neo was Loxed and excised by Cre recombinase, which led to higher tissue levels of Met(grb2) protein, sufficient to rescue viability. In Met(grb2/grb2neo-) mice the size of the cerebellum was reduced and foliation defects were evident, especially in the central and posterior half of the vermis. Proliferation of granule precursors in vivo was 25% lower than in controls. In cultures of mutant granule cells HGF-induced microtubule-associated protein kinase activation was reduced and transient. Behavioral tests indicated a balance impairment in Met(grb2/grb2neo-) mice. Altogether these data indicate that normal cerebellar development and, possibly, function, require HGF and Met, and that proliferation of granule cells in the cerebellum critically depends on full HGF/Met signaling.
Figures
Similar articles
-
Neuroprotection by scatter factor/hepatocyte growth factor and FGF-1 in cerebellar granule neurons is phosphatidylinositol 3-kinase/akt-dependent and MAPK/CREB-independent.J Neurochem. 2002 Apr;81(2):365-78. doi: 10.1046/j.1471-4159.2002.00837.x. J Neurochem. 2002. PMID: 12064484
-
Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling.J Neurosci. 2008 Jun 4;28(23):5901-9. doi: 10.1523/JNEUROSCI.1083-08.2008. J Neurosci. 2008. PMID: 18524894 Free PMC article.
-
Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway.Cell Signal. 2008 May;20(5):825-35. doi: 10.1016/j.cellsig.2007.12.013. Epub 2007 Dec 27. Cell Signal. 2008. PMID: 18262389 Free PMC article.
-
HGF: a multifunctional growth factor controlling cell scattering.Int J Biochem Cell Biol. 1999 Dec;31(12):1357-62. doi: 10.1016/s1357-2725(99)00089-8. Int J Biochem Cell Biol. 1999. PMID: 10641789 Review.
-
c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention.Cancer Lett. 2005 Jul 8;225(1):1-26. doi: 10.1016/j.canlet.2004.09.044. Epub 2004 Nov 11. Cancer Lett. 2005. PMID: 15922853 Review.
Cited by
-
The Cerebellar Involvement in Autism Spectrum Disorders: From the Social Brain to Mouse Models.Int J Mol Sci. 2022 Mar 31;23(7):3894. doi: 10.3390/ijms23073894. Int J Mol Sci. 2022. PMID: 35409253 Free PMC article. Review.
-
Association of genetic variation in the MET proto-oncogene with schizophrenia and general cognitive ability.Am J Psychiatry. 2010 Apr;167(4):436-43. doi: 10.1176/appi.ajp.2009.09050615. Epub 2010 Jan 15. Am J Psychiatry. 2010. PMID: 20080979 Free PMC article.
-
MET receptor tyrosine kinase as an autism genetic risk factor.Int Rev Neurobiol. 2013;113:135-65. doi: 10.1016/B978-0-12-418700-9.00005-8. Int Rev Neurobiol. 2013. PMID: 24290385 Free PMC article. Review.
-
HGF-Met Pathway in Regeneration and Drug Discovery.Biomedicines. 2014 Oct 31;2(4):275-300. doi: 10.3390/biomedicines2040275. Biomedicines. 2014. PMID: 28548072 Free PMC article. Review.
-
Immune mediators in the brain and peripheral tissues in autism spectrum disorder.Nat Rev Neurosci. 2015 Aug;16(8):469-86. doi: 10.1038/nrn3978. Nat Rev Neurosci. 2015. PMID: 26189694 Free PMC article. Review.
References
-
- Wechsler-Reya R. J. & Scott, M. P. (1999) Neuron 22, 103-114. - PubMed
-
- Wallace V. A. (1999) Curr. Biol. 9, 445-448. - PubMed
-
- Dahmane N. & Ruiz-i-Altaba, A. (1999) Development (Cambridge, U.K.) 126, 3089-3100. - PubMed
-
- Bates B., Rios, B., Trumpp, A., Chen, C., Fan, G., Bishop, J. M. & Jaenisch, R. (1999) Nat. Neurosci. 2, 115-117. - PubMed
-
- Schwartz P. M, Borghesani, P. R., Levy, R. L., Pomeroy, S. L. & Segal, R. A. (1997) Neuron 19, 269-281. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

