Mycothiol-deficient Mycobacterium smegmatis mutants are hypersensitive to alkylating agents, free radicals, and antibiotics
- PMID: 12384335
- PMCID: PMC128700
- DOI: 10.1128/AAC.46.11.3348-3355.2002
Mycothiol-deficient Mycobacterium smegmatis mutants are hypersensitive to alkylating agents, free radicals, and antibiotics
Abstract
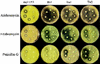
Mycothiol (MSH; 1D-myo-inosityl 2-[N-acetyl-L-cysteinyl]amido-2-deoxy-alpha-D-glucopyranoside) is the major low-molecular-weight thiol produced by mycobacteria. Mutants of Mycobacterium smegmatis mc(2)155 deficient in MSH production were produced by chemical mutagenesis as well as by transposon mutagenesis. One chemical mutant (mutant I64) and two transposon mutants (mutants Tn1 and Tn2) stably deficient in MSH production were isolated by screening for reduced levels of MSH content. The MSH contents of transposon mutants Tn1 and Tn2 were found to be less than 0.1% that of the parent strain, and the MSH content of I64 was found to be 1 to 5% that of the parent strain. All three strains accumulated 1D-myo-inosityl 2-deoxy-alpha-D-glucopyranoside to levels 20- to 25-fold the level found in the parent strain. The cysteine:1D-myo-inosityl 2-amino-2-deoxy-alpha-D-glucopyranoside ligase (MshC) activities of the three mutant strains were < or =2% that of the parent strain. Phenotypic analysis revealed that these MSH-deficient mutants possess increased susceptibilities to free radicals and alkylating agents and to a wide range of antibiotics including erythromycin, azithromycin, vancomycin, penicillin G, rifamycin, and rifampin. Conversely, the mutants possess at least 200-fold higher levels of resistance to isoniazid than the wild type. We mapped the mutation in the chemical mutant by sequencing the mshC gene and showed that a single amino acid substitution (L205P) is responsible for reduced MSH production and its associated phenotype. Our results demonstrate that there is a direct correlation between MSH depletion and enhanced sensitivity to toxins and antibiotics.
Figures



Similar articles
-
Characterization of Mycobacterium smegmatis mutants defective in 1-d-myo-inosityl-2-amino-2-deoxy-alpha-d-glucopyranoside and mycothiol biosynthesis.Biochem Biophys Res Commun. 1999 Feb 16;255(2):239-44. doi: 10.1006/bbrc.1999.0156. Biochem Biophys Res Commun. 1999. PMID: 10049692
-
Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics.Mol Microbiol. 2003 Mar;47(6):1723-32. doi: 10.1046/j.1365-2958.2003.03416.x. Mol Microbiol. 2003. PMID: 12622824
-
Inactivation of mshB, a key gene in the mycothiol biosynthesis pathway in Mycobacterium smegmatis.Microbiology (Reading). 2003 May;149(Pt 5):1341-1349. doi: 10.1099/mic.0.26084-0. Microbiology (Reading). 2003. PMID: 12724395
-
Mycothiol biochemistry.Arch Microbiol. 2002 Dec;178(6):388-94. doi: 10.1007/s00203-002-0469-4. Epub 2002 Sep 3. Arch Microbiol. 2002. PMID: 12420157 Review.
-
Chemistry and Redox Biology of Mycothiol.Antioxid Redox Signal. 2018 Feb 20;28(6):487-504. doi: 10.1089/ars.2017.7074. Epub 2017 May 10. Antioxid Redox Signal. 2018. PMID: 28372502 Review.
Cited by
-
Ancestral antibiotic resistance in Mycobacterium tuberculosis.Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12200-5. doi: 10.1073/pnas.0505446102. Epub 2005 Aug 15. Proc Natl Acad Sci U S A. 2005. PMID: 16103351 Free PMC article.
-
Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria.Microbiol Mol Biol Rev. 2008 Sep;72(3):471-94. doi: 10.1128/MMBR.00008-08. Microbiol Mol Biol Rev. 2008. PMID: 18772286 Free PMC article. Review.
-
Targeting Mycobacterium tuberculosis Sensitivity to Thiol Stress at Acidic pH Kills the Bacterium and Potentiates Antibiotics.Cell Chem Biol. 2017 Aug 17;24(8):993-1004.e4. doi: 10.1016/j.chembiol.2017.06.018. Epub 2017 Aug 3. Cell Chem Biol. 2017. PMID: 28781126 Free PMC article.
-
Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes.Antimicrob Agents Chemother. 2006 Aug;50(8):2836-41. doi: 10.1128/AAC.00295-06. Antimicrob Agents Chemother. 2006. PMID: 16870781 Free PMC article.
-
Functional characterization of Corynebacterium glutamicum mycothiol S-conjugate amidase.PLoS One. 2014 Dec 16;9(12):e115075. doi: 10.1371/journal.pone.0115075. eCollection 2014. PLoS One. 2014. PMID: 25514023 Free PMC article.
References
-
- Anderberg, S., G. L. Newton, and R. C. Fahey. 1998. Mycothiol biosynthesis and metabolism: cellular levels of potential intermediates in the biosynthesis and degradation of mycothiol. J. Biol. Chem. 273:30391-30397. - PubMed
-
- Aoyama, T., W. Zhao, F. Kojima, Y. Muraoka, H. Maganawa, T. Takeuchi, and T. Aoyagi. 1993. Cysfluoretin, a new inhibitor of glutathione S-transferase, produced by Streptomyces sp. MI384-DF12. J. Antibiot. 46:1471. - PubMed
-
- Carney, J. R., S.-T. Hong, and S. J. Gould. 1997. Seongomycin: a new sulfur containing benzo[b]fluorene derived from genes clustered with those for kinamycin biosynthesis. Tetrahedron Lett. 38:3139-3142.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

