Inotropic responses to human gene 2 (B29) relaxin in a rat model of myocardial infarction (MI): effect of pertussis toxin
- PMID: 12381685
- PMCID: PMC1573542
- DOI: 10.1038/sj.bjp.0704922
Inotropic responses to human gene 2 (B29) relaxin in a rat model of myocardial infarction (MI): effect of pertussis toxin
Abstract
Relaxin produces powerful inotropic and chronotropic responses in isolated atria. The effect of relaxin has been examined in a rat model of cardiac failure, induced by myocardial infarction (MI). Maximum inotropic responses to isoprenaline (sham 5.4+/-0.3 mN; MI 2.6+/-0.3 mN; P<0.001) and relaxin (sham 5.1+/-0.6 mN; MI 2.8+/-0.5 mN; P=0.013) were reduced in left atria following MI. No change in chronotropic responsiveness was observed in right atria. Pertussis toxin (PTX) treatment restored inotropic responses to isoprenaline (sham 5.5+/-1.3 mN; MI 5.8+/-1.0 mN; P=0.850) but not to relaxin. Instead, PTX reduced inotropic responses to relaxin in sham animals to the same level seen in the MI group (sham 3.2+/-1.7 mN; MI 2.8+/-0.6 mN; P=0.847). In right atria, PTX treatment did not affect the maximum chronotropic response to isoprenaline, but reduced responses to relaxin in both sham and MI animals. R3 relaxin and relaxin receptor (LGR7) mRNA was present in atria and left ventricle (LV) from sham and MI animals. R3 relaxin mRNA expression was increased in atria but not LV from MI animals. LGR7 mRNA expression was reduced in atria and LV from MI animals. PTX treatment in unoperated rats increased chronotropic responses (vehicle 184.3+/-5.3 beats min(-1); PTX 211.3+/-9.5 beats min(-1); P=0.029) and produced a rightward shift in the concentration-response curve to isoprenaline in left atria. PTX reduced inotropic (vehicle 3.3+/-0.7 mN; PTX 0.8+/-0.2 mN; P=0.005) and chronotropic (vehicle 130.2+/-8.1 beats min(-1); PTX 90.6+/-11.1 beats min(-1); P=0.012) responses to relaxin. 6 In left atria, relaxin produced a small increase in cAMP compared to those produced by isoprenaline and forskolin. However, PTX treatment significantly reduced relaxin-, isoprenaline- and forskolin-stimulated cAMP accumulation. Cardiac failure in MI animals caused a reduced inotropic response to both relaxin and (-)-isoprenaline. In non-MI animals, PTX treatment also reduced inotropic responses to relaxin. Differences between responses to (-)-isoprenaline and relaxin can be explained by changes in coupling efficiency occurring at the level of adenylate cyclase.
Figures
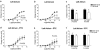
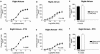
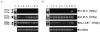
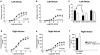
Similar articles
-
Desensitization and resensitization of beta 1- and putative beta 4-adrenoceptor mediated responses occur in parallel in a rat model of cardiac failure.Br J Pharmacol. 1999 Dec;128(7):1399-406. doi: 10.1038/sj.bjp.0702920. Br J Pharmacol. 1999. PMID: 10602318 Free PMC article.
-
Desensitization of cardiac beta-adrenoceptor signaling with heart failure produced by myocardial infarction in the rat. Evidence for the role of Gi but not Gs or phosphorylating proteins.J Mol Cell Cardiol. 1999 Jun;31(6):1185-201. doi: 10.1006/jmcc.1999.0951. J Mol Cell Cardiol. 1999. PMID: 10371694
-
Cardiostimulant and cardiodepressant effects through overexpressed human beta2-adrenoceptors in murine heart: regional differences and functional role of beta1-adrenoceptors.Naunyn Schmiedebergs Arch Pharmacol. 2003 Apr;367(4):380-90. doi: 10.1007/s00210-002-0681-4. Epub 2003 Mar 4. Naunyn Schmiedebergs Arch Pharmacol. 2003. PMID: 12690430
-
Relaxin: more than just a hormone of pregnancy.Trends Pharmacol Sci. 1993 Jan;14(1):4-6. doi: 10.1016/0165-6147(93)90105-s. Trends Pharmacol Sci. 1993. PMID: 8382887 Review. No abstract available.
-
Relaxin-2 as a Potential Biomarker in Cardiovascular Diseases.J Pers Med. 2022 Jun 21;12(7):1021. doi: 10.3390/jpm12071021. J Pers Med. 2022. PMID: 35887517 Free PMC article. Review.
Cited by
-
Constitutive formation of an RXFP1-signalosome: a novel paradigm in GPCR function and regulation.Br J Pharmacol. 2012 Mar;165(6):1644-1658. doi: 10.1111/j.1476-5381.2011.01470.x. Br J Pharmacol. 2012. PMID: 21557732 Free PMC article. Review.
-
Relaxin-2 in Cardiometabolic Diseases: Mechanisms of Action and Future Perspectives.Front Physiol. 2017 Aug 18;8:599. doi: 10.3389/fphys.2017.00599. eCollection 2017. Front Physiol. 2017. PMID: 28868039 Free PMC article. Review.
-
Rat Models of Cardiorenal Syndrome and Methods for Functional Assessment.Methods Mol Biol. 2024;2803:145-162. doi: 10.1007/978-1-0716-3846-0_11. Methods Mol Biol. 2024. PMID: 38676891
-
Upregulation of relaxin after experimental subarachnoid hemorrhage in rabbits.Biomed Res Int. 2014;2014:836397. doi: 10.1155/2014/836397. Epub 2014 Jul 16. Biomed Res Int. 2014. PMID: 25133183 Free PMC article.
-
Relaxin-2 Prevents Erectile Dysfunction by Cavernous Nerve, Endothelial and Histopathological Protection Effects in Rats with Bilateral Cavernous Nerve Injury.World J Mens Health. 2023 Apr;41(2):434-445. doi: 10.5534/wjmh.220003. Epub 2022 Jul 14. World J Mens Health. 2023. PMID: 36047071 Free PMC article.
References
-
- BARTSCH O., BARTLICK B., IVELL R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol. Human Reprod. 2001;7:799–809. - PubMed
-
- BATHGATE R.A.D., SAMUEL C.S., BURAZIN T.C.D., LAYFIELD S., CLAASZ A.A., REYTOMAS I.G.T., DAWSON N.F., ZHAO C., BOND C., SUMMERS R.J., PARRY L.J., WADE J.D., TREGEAR G.W. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene: Novel members of the relaxin peptide family. J. Biol. Chem. 2002;277:1148–1157. - PubMed
-
- BURAZIN T.C.D., BATHGATE R.A.D., MACRIS M., LAYFIELD S., GUNDLACH A.L., TREGEAR G.W.Restricted, but abundant, expression of the novel rat gene-3 (R3) relaxin in the dorsal tegmental region of brain J. Neurochem. 2002. in press - PubMed
-
- CARRELL D.T., PETERSON C.M., URRY R.L. The binding of recombinant human relaxin to human spermatozoa. Endocrin. Res. 1995;21:697–707. - PubMed
-
- CHENG H.C., KEMP B.E., PEARSON R.B., SMITH A.J., MISCONI L., VAN PATTEN S.M., WALSH D.A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J. Biol. Chem. 1986;261:989–992. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

