Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression
- PMID: 12379669
- PMCID: PMC130313
- DOI: 10.1128/IAI.70.11.5946-5954.2002
Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression
Abstract
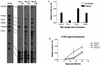
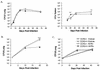
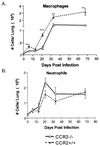
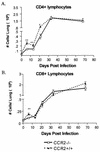
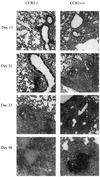
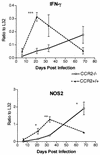
Within a Mycobacterium tuberculosis-induced granuloma, lymphocytes and macrophages work together to control bacterial growth and limit the spread of infection. Chemokines and chemokine receptors are involved in cell migration and are logical candidates for a role in granuloma formation. In the present study we addressed the role of CC chemokine receptor 2 (CCR2) in M. tuberculosis infection. In previous studies (W. Peters et al., Proc. Natl. Acad. Sci. USA 98:7958-7963, 2001), CCR2(-/-) mice were found to be highly susceptible to a moderate or high dose of H37Rv administered intravenously (i.v.). We have expanded those studies to demonstrate that the susceptibility of CCR2(-/-) mice is dose dependent. After low-dose aerosol or i.v. infection of CCR2(-/-) mice with M. tuberculosis, there was a substantial delay in cell migration to the lungs and delayed expression of gamma interferon and inducible nitric oxide synthase. The CCR2(-/-) mice had a severe and prolonged deficiency in the number of macrophages in the lungs and an early increase in the number of neutrophils. Despite these deficiencies in cell migration, the CCR2(-/-) mice did not have increased bacterial loads in the lungs compared to wild-type (C57BL/6) mice and successfully formed granulomas. This finding is in contrast to CCR2(-/-) mice infected with a high dose of M. tuberculosis administered i.v. These results indicate that with low-dose infection, a delay in immune response in the lungs does not necessarily have detrimental long-term effects on the progression of the disease. The fact that CCR2(-/-) mice survive with substantially fewer macrophages in the low-dose models implies that the immune response to low-dose M. tuberculosis infection in mice is more robust than necessary to control the infection. Finally, these data demonstrate that, in cases of infectious disease in knockout models, clear phenotypes may not be evident when one is solely evaluating bacterial numbers and survival. Functional assays may be necessary to reveal roles for components of the multifactorial immune system.
Figures
Similar articles
-
Widespread bronchogenic dissemination makes DBA/2 mice more susceptible than C57BL/6 mice to experimental aerosol infection with Mycobacterium tuberculosis.Infect Immun. 2003 Oct;71(10):5845-54. doi: 10.1128/IAI.71.10.5845-5854.2003. Infect Immun. 2003. PMID: 14500506 Free PMC article.
-
Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice.J Clin Invest. 1997 Nov 15;100(10):2552-61. doi: 10.1172/JCI119798. J Clin Invest. 1997. PMID: 9366570 Free PMC article.
-
The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes.J Immunol. 2003 May 15;170(10):5210-8. doi: 10.4049/jimmunol.170.10.5210. J Immunol. 2003. PMID: 12734369
-
Involvement of chemokine receptor 2 and its ligand, monocyte chemoattractant protein-1, in the development of atherosclerosis: lessons from knockout mice.Curr Opin Lipidol. 2001 Apr;12(2):175-80. doi: 10.1097/00041433-200104000-00011. Curr Opin Lipidol. 2001. PMID: 11264989 Review.
-
Recent advances targeting C-C chemokine receptor type 2 for liver diseases in monocyte/macrophage.Liver Int. 2020 Dec;40(12):2928-2936. doi: 10.1111/liv.14687. Epub 2020 Oct 21. Liver Int. 2020. PMID: 33025657 Review.
Cited by
-
Lipocalin 2 regulates inflammation during pulmonary mycobacterial infections.PLoS One. 2012;7(11):e50052. doi: 10.1371/journal.pone.0050052. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185529 Free PMC article.
-
The immunological life cycle of tuberculosis.Nat Rev Immunol. 2012 Jul 13;12(8):581-91. doi: 10.1038/nri3259. Nat Rev Immunol. 2012. PMID: 22790178 Review.
-
Recruited monocytes/macrophages drive pulmonary neutrophilic inflammation and irreversible lung tissue remodeling in cystic fibrosis.Cell Rep. 2022 Dec 13;41(11):111797. doi: 10.1016/j.celrep.2022.111797. Cell Rep. 2022. PMID: 36516754 Free PMC article.
-
Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis.J Immunol. 2011 Jan 1;186(1):471-8. doi: 10.4049/jimmunol.1003003. Epub 2010 Dec 3. J Immunol. 2011. PMID: 21131425 Free PMC article.
-
IP-10 and MIG are compartmentalized at the site of disease during pleural and meningeal tuberculosis and are decreased after antituberculosis treatment.Clin Vaccine Immunol. 2014 Dec;21(12):1635-44. doi: 10.1128/CVI.00499-14. Epub 2014 Oct 1. Clin Vaccine Immunol. 2014. PMID: 25274803 Free PMC article.
References
-
- Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. - PubMed
-
- Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

