The influenza A virus NS1 protein inhibits activation of Jun N-terminal kinase and AP-1 transcription factors
- PMID: 12368362
- PMCID: PMC136597
- DOI: 10.1128/jvi.76.21.11166-11171.2002
The influenza A virus NS1 protein inhibits activation of Jun N-terminal kinase and AP-1 transcription factors
Abstract
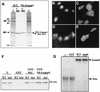
The influenza A virus nonstructural NS1 protein is known to modulate host cell gene expression and to inhibit double-stranded RNA (dsRNA)-mediated antiviral responses. Here we identify NS1 as the first viral protein that antagonizes virus- and dsRNA-induced activation of the stress response-signaling pathway mediated through Jun N-terminal kinase.
Figures

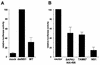

Similar articles
-
Double-stranded RNA-induced activation of activating protein-1 promoter is differentially regulated by the non-structural protein 1 of avian influenza A viruses.Viral Immunol. 2012 Feb;25(1):79-85. doi: 10.1089/vim.2011.0059. Epub 2012 Jan 12. Viral Immunol. 2012. PMID: 22239235 Free PMC article.
-
Activation of c-jun N-terminal kinase upon influenza A virus (IAV) infection is independent of pathogen-related receptors but dependent on amino acid sequence variations of IAV NS1.J Virol. 2014 Aug;88(16):8843-52. doi: 10.1128/JVI.00424-14. Epub 2014 May 28. J Virol. 2014. PMID: 24872593 Free PMC article.
-
Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus.J Virol. 2007 Jan;81(2):514-24. doi: 10.1128/JVI.01265-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079289 Free PMC article.
-
The influenza virus NS1 protein as a therapeutic target.Antiviral Res. 2013 Sep;99(3):409-16. doi: 10.1016/j.antiviral.2013.06.005. Epub 2013 Jun 21. Antiviral Res. 2013. PMID: 23796981 Free PMC article. Review.
-
Structure and Function of the Influenza A Virus Non-Structural Protein 1.J Microbiol Biotechnol. 2019 Aug 28;29(8):1184-1192. doi: 10.4014/jmb.1903.03053. J Microbiol Biotechnol. 2019. PMID: 31154753 Review.
Cited by
-
Differential requirement for c-Jun N-terminal kinase 1 in lung inflammation and host defense.PLoS One. 2012;7(4):e34638. doi: 10.1371/journal.pone.0034638. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514650 Free PMC article.
-
The RNA binding domain of influenza A virus NS1 protein affects secretion of tumor necrosis factor alpha, interleukin-6, and interferon in primary murine tracheal epithelial cells.J Virol. 2007 Sep;81(17):9469-80. doi: 10.1128/JVI.00989-07. Epub 2007 Jun 27. J Virol. 2007. PMID: 17596305 Free PMC article.
-
The Two Sides of the Same Coin-Influenza Virus and Intracellular Signal Transduction.Cold Spring Harb Perspect Med. 2021 Jan 4;11(1):a038513. doi: 10.1101/cshperspect.a038513. Cold Spring Harb Perspect Med. 2021. PMID: 31871235 Free PMC article. Review.
-
The N- and C-terminal domains of the NS1 protein of influenza B virus can independently inhibit IRF-3 and beta interferon promoter activation.J Virol. 2004 Nov;78(21):11574-82. doi: 10.1128/JVI.78.21.11574-11582.2004. J Virol. 2004. PMID: 15479798 Free PMC article.
-
Apoptosis, cytokine and chemokine induction by non-structural 1 (NS1) proteins encoded by different influenza subtypes.Virol J. 2011 Dec 21;8:554. doi: 10.1186/1743-422X-8-554. Virol J. 2011. PMID: 22185562 Free PMC article.
References
-
- Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-351. In D. M. Knipe, P. M. Howley, D. E. Griffin et al. (ed.), Fields virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
-
- Chu, W. M., D. Ostertag, Z. W. Li, L. Chang, Y. Chen, Y. Hu, B. Williams, J. Perrault, and M. Karin. 1999. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11:721-731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

