Furin at the cutting edge: from protein traffic to embryogenesis and disease
- PMID: 12360192
- PMCID: PMC1964754
- DOI: 10.1038/nrm934
Furin at the cutting edge: from protein traffic to embryogenesis and disease
Abstract
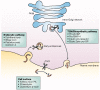
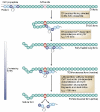
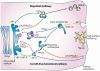
Furin catalyses a simple biochemical reaction--the proteolytic maturation of proprotein substrates in the secretory pathway. But the simplicity of this reaction belies furin's broad and important roles in homeostasis, as well as in diseases ranging from Alzheimer's disease and cancer to anthrax and Ebola fever. This review summarizes various features of furin--its structural and enzymatic properties, intracellular localization, trafficking, substrates, and roles in vivo.
Figures




Similar articles
-
Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis.Trends Cell Biol. 1999 Jan;9(1):28-35. doi: 10.1016/s0962-8924(98)01382-8. Trends Cell Biol. 1999. PMID: 10087614 Review.
-
Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway.J Cell Biol. 1997 Dec 29;139(7):1719-33. doi: 10.1083/jcb.139.7.1719. J Cell Biol. 1997. PMID: 9412467 Free PMC article.
-
The crystal structure of the proprotein processing proteinase furin explains its stringent specificity.Nat Struct Biol. 2003 Jul;10(7):520-6. doi: 10.1038/nsb941. Nat Struct Biol. 2003. PMID: 12794637
-
Activation of membrane-type matrix metalloproteinase 3 zymogen by the proprotein convertase furin in the trans-Golgi network.Cancer Res. 2002 Feb 1;62(3):675-81. Cancer Res. 2002. PMID: 11830519
-
Structure and function of eukaryotic proprotein processing enzymes of the subtilisin family of serine proteases.Crit Rev Oncog. 1993;4(2):115-36. Crit Rev Oncog. 1993. PMID: 8420571 Review.
Cited by
-
Regulation of ADAM10 activity through microdomain-dependent intracellular calcium changes.Cell Commun Signal. 2024 Nov 4;22(1):531. doi: 10.1186/s12964-024-01891-5. Cell Commun Signal. 2024. PMID: 39497138 Free PMC article.
-
Cytomegalovirus Generates Assembly Compartment in the Early Phase of Infection by Perturbation of Host-Cell Factors Recruitment at the Early Endosome/Endosomal Recycling Compartment/Trans-Golgi Interface.Front Cell Dev Biol. 2020 Sep 11;8:563607. doi: 10.3389/fcell.2020.563607. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33042998 Free PMC article.
-
Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space.Cells. 2021 Feb 26;10(3):503. doi: 10.3390/cells10030503. Cells. 2021. PMID: 33652973 Free PMC article. Review.
-
SARS-CoV-2 cellular tropism and direct multiorgan failure in COVID-19 patients: Bioinformatic predictions, experimental observations, and open questions.Cell Biol Int. 2023 Feb;47(2):308-326. doi: 10.1002/cbin.11928. Epub 2022 Oct 13. Cell Biol Int. 2023. PMID: 36229927 Free PMC article. Review.
-
Protection of the Furin Cleavage Site in Low-Toxicity Immunotoxins Based on Pseudomonas Exotoxin A.Toxins (Basel). 2016 Jul 25;8(8):217. doi: 10.3390/toxins8080217. Toxins (Basel). 2016. PMID: 27463727 Free PMC article.
References
-
- Seidah NG, Day R, Marcinkiewicz M, Chretien M. Precursor convertases: an evolutionary ancient, cell-specific, combinatorial mechanism yielding diverse bioactive peptides and proteins. Ann. N. Y. Acad. Sci. 1998;839:9–24. - PubMed
-
- Thacker C, Rose AM. A look at the Caenorhabditis elegans Kex2/Subtilisin-like proprotein convertase family. Bioessays. 2000;22:545–553. - PubMed
-
- Zhou A, Martin S, Lipkind G, LaMendola J, Steiner DF. Regulatory roles of the P domain of the subtilisin-like prohormone convertases. J. Biol. Chem. 1998;273:11107–11114. - PubMed
-
- Siezen RJ, Creemers JW, Van de Ven WJ. Homology modelling of the catalytic domain of human furin. A model for the eukaryotic subtilisin-like proprotein convertases. Eur. J. Biochem. 1994;222:255–266. - PubMed
-
- Rockwell NC, Fuller RS. Specific modulation of Kex2/Furin family proteases by potassium. J. Biol. Chem. 2002;277:17531–17537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

