Sendai virus trailer RNA binds TIAR, a cellular protein involved in virus-induced apoptosis
- PMID: 12356730
- PMCID: PMC129035
- DOI: 10.1093/emboj/cdf513
Sendai virus trailer RNA binds TIAR, a cellular protein involved in virus-induced apoptosis
Abstract
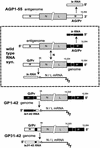
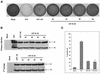
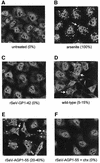
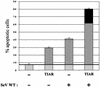
Sendai virus (SeV) leader (le) and trailer (tr) RNAs are short transcripts generated during abortive antigenome and genome synthesis, respectively. Recom binant SeV (rSeV) that express tr-like RNAs from the leader region are non-cytopathic and, moreover, prevent wild-type SeV from inducing apoptosis in mixed infections. These rSeV thus appear to have gained a function. Here we report that tr RNA binds to a cellular protein with many links to apoptosis (TIAR) via the AU-rich sequence 5' UUUUAAAUUUU. Duplication of this AU-rich sequence alone within the le RNA confers TIAR binding on this le* RNA and a non-cytopathic phenotype to these rSeV in cell culture. Transgenic overexpression of TIAR during SeV infection promotes apoptosis and reverses the anti-apoptotic effects of le* RNA expression. More over, TIAR overexpression and SeV infection act synergistically to induce apoptosis. These short viral RNAs may act by sequestering TIAR, a multivalent RNA recognition motif (RRM) family RNA-binding protein involved in SeV-induced apoptosis. In this view, tr RNA is not simply a by-product of abortive genome synthesis, but is also an antigenome transcript that modulates the cellular antiviral response.
Figures




Similar articles
-
Sendai virus trailer RNA simultaneously blocks two apoptosis-inducing mechanisms in a cell type-dependent manner.J Gen Virol. 2005 Aug;86(Pt 8):2305-2314. doi: 10.1099/vir.0.81022-0. J Gen Virol. 2005. PMID: 16033978
-
The short Sendai virus leader region controls induction of programmed cell death.Virology. 1998 Apr 10;243(2):340-53. doi: 10.1006/viro.1998.9063. Virology. 1998. PMID: 9568033
-
[Comparison of the rescue efficiency of Sendai virus minigenome mediated by CMV and T7 promoter].Bing Du Xue Bao. 2012 May;28(3):237-45. Bing Du Xue Bao. 2012. PMID: 22764526 Chinese.
-
Sendai Virus and a Unified Model of Mononegavirus RNA Synthesis.Viruses. 2021 Dec 9;13(12):2466. doi: 10.3390/v13122466. Viruses. 2021. PMID: 34960735 Free PMC article. Review.
-
[Sendai virus proteins counteracting the host innate immunity].Uirusu. 2004 Dec;54(2):179-88. doi: 10.2222/jsv.54.179. Uirusu. 2004. PMID: 15745155 Review. Japanese.
Cited by
-
Respiratory Syncytial Virus and Cellular Stress Responses: Impact on Replication and Physiopathology.Viruses. 2016 May 12;8(5):124. doi: 10.3390/v8050124. Viruses. 2016. PMID: 27187445 Free PMC article. Review.
-
Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation.Mol Biol Cell. 2005 Aug;16(8):3753-63. doi: 10.1091/mbc.e05-02-0124. Epub 2005 Jun 1. Mol Biol Cell. 2005. PMID: 15930128 Free PMC article.
-
Strategies for Success. Viral Infections and Membraneless Organelles.Front Cell Infect Microbiol. 2019 Oct 11;9:336. doi: 10.3389/fcimb.2019.00336. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31681621 Free PMC article. Review.
-
Stress granules: potential therapeutic targets for infectious and inflammatory diseases.Front Immunol. 2023 May 2;14:1145346. doi: 10.3389/fimmu.2023.1145346. eCollection 2023. Front Immunol. 2023. PMID: 37205103 Free PMC article. Review.
-
The cryoEM structure of the Hendra henipavirus nucleoprotein reveals insights into paramyxoviral nucleocapsid architectures.Sci Rep. 2024 Jun 18;14(1):14099. doi: 10.1038/s41598-024-58243-z. Sci Rep. 2024. PMID: 38890308 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

