Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1
- PMID: 12239292
- PMCID: PMC136556
- DOI: 10.1128/jvi.76.20.10177-10187.2002
Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1
Abstract
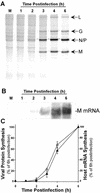
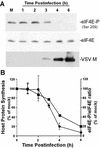
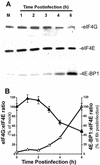
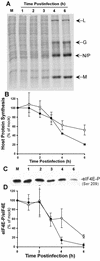
Vesicular stomatitis virus (VSV) modulates protein synthesis in infected cells in a way that allows the translation of its own 5'-capped mRNA but inhibits the translation of host mRNA. Previous data have shown that inactivation of eIF2alpha is important for VSV-induced inhibition of host protein synthesis. We tested whether there is a role for eIF4F in this inhibition. The multisubunit eIF4F complex is involved in the regulation of protein synthesis via phosphorylation of cap-binding protein eIF4E, a subunit of eIF4F. Translation of host mRNA is significantly reduced under conditions in which eIF4E is dephosphorylated. To determine whether VSV infection alters the eIF4F complex, we analyzed eIF4E phosphorylation and the association of eIF4E with other translation initiation factors, such as eIF4G and the translation inhibitor 4E-BP1. VSV infection of HeLa cells resulted in the dephosphorylation of eIF4E at serine 209 between 3 and 6 h postinfection. This time course corresponded well to that of the inhibition of host protein synthesis induced by VSV infection. Cells infected with a VSV mutant that is delayed in the ability to inhibit host protein synthesis were also delayed in dephosphorylation of eIF4E. In addition to decreasing eIF4E phosphorylation, VSV infection also resulted in the dephosphorylation and activation of eIF4E-binding protein 4E-BP1 between 3 and 6 h postinfection. Analysis of cap-binding complexes showed that VSV infection reduced the association of eIF4E with the eIF4G scaffolding subunit at the same time as its association with 4E-BP1 increased and that these time courses correlated with the dephosphorylation of eIF4E. These changes in the eIF4F complex occurred over the same time period as the onset of viral protein synthesis, suggesting that activation of 4E-BP1 does not inhibit translation of viral mRNAs. In support of this idea, VSV protein synthesis was not affected by the presence of rapamycin, a drug that blocks 4E-BP1 phosphorylation. These data show that VSV infection results in modifications of the eIF4F complex that are correlated with the inhibition of host protein synthesis and that translation of VSV mRNAs occurs despite lowered concentrations of the active cap-binding eIF4F complex. This is the first noted modification of both eIF4E and 4E-BP1 phosphorylation levels among viruses that produce capped mRNA for protein translation.
Figures



Similar articles
-
Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus.Proc Natl Acad Sci U S A. 1996 May 28;93(11):5578-83. doi: 10.1073/pnas.93.11.5578. Proc Natl Acad Sci U S A. 1996. PMID: 8643618 Free PMC article.
-
Repressor binding to a dorsal regulatory site traps human eIF4E in a high cap-affinity state.EMBO J. 1999 Jul 15;18(14):4068-75. doi: 10.1093/emboj/18.14.4068. EMBO J. 1999. PMID: 10406811 Free PMC article.
-
Nitric oxide mediates NMDA-induced persistent inhibition of protein synthesis through dephosphorylation of eukaryotic initiation factor 4E-binding protein 1 and eukaryotic initiation factor 4G proteolysis.Biochem J. 2008 May 1;411(3):667-77. doi: 10.1042/BJ20071060. Biochem J. 2008. PMID: 18215131
-
Manipulation of the host translation initiation complex eIF4F by DNA viruses.Biochem Soc Trans. 2010 Dec;38(6):1511-6. doi: 10.1042/BST0381511. Biochem Soc Trans. 2010. PMID: 21118117 Review.
-
Regulation of translation initiation by amino acids in eukaryotic cells.Prog Mol Subcell Biol. 2001;26:155-84. doi: 10.1007/978-3-642-56688-2_6. Prog Mol Subcell Biol. 2001. PMID: 11575165 Review.
Cited by
-
The VSV matrix protein inhibits NF-κB and the interferon response independently in mouse L929 cells.Virology. 2020 Sep;548:117-123. doi: 10.1016/j.virol.2020.06.013. Epub 2020 Jun 29. Virology. 2020. PMID: 32838932 Free PMC article.
-
IRES-mediated translation of foot-and-mouth disease virus (FMDV) in cultured cells derived from FMDV-susceptible and -insusceptible animals.BMC Vet Res. 2016 Mar 31;12:66. doi: 10.1186/s12917-016-0694-8. BMC Vet Res. 2016. PMID: 27036295 Free PMC article.
-
Phospho-eIF4E stimulation regulates coronavirus entry by selective expression of cell membrane-residential factors.J Virol. 2024 Feb 20;98(2):e0194823. doi: 10.1128/jvi.01948-23. Epub 2024 Feb 1. J Virol. 2024. PMID: 38299843 Free PMC article.
-
The poly(A) binding protein is internalized in virus-induced vesicles or redistributed to the nucleolus during turnip mosaic virus infection.J Virol. 2007 Oct;81(20):10905-13. doi: 10.1128/JVI.01243-07. Epub 2007 Aug 1. J Virol. 2007. PMID: 17670821 Free PMC article.
-
Vaccinia Virus as a Master of Host Shutoff Induction: Targeting Processes of the Central Dogma and Beyond.Pathogens. 2020 May 21;9(5):400. doi: 10.3390/pathogens9050400. Pathogens. 2020. PMID: 32455727 Free PMC article. Review.
References
-
- Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. - PubMed
-
- Berg, D. T., and B. W. Grinnell. 1992. 5′ sequence of vesicular stomatitis virus N-gene confers selective translation of mRNA. Biochem. Biophys. Res. Commun. 189:1585-1590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

