Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo
- PMID: 12235214
- PMCID: PMC2194052
- DOI: 10.1084/jem.20020295
Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo
Abstract
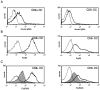
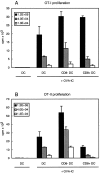
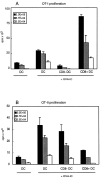
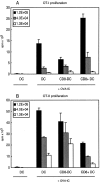
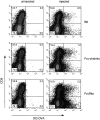
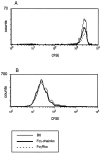
Murine splenic dendritic cells (DCs) can be divided into two subsets based on CD8alpha expression, but the specific role of each subset in stimulation of T cells is largely unknown. An important function of DCs is the ability to take up exogenous antigens and cross-present them in the context of major histocompatibility complex (MHC) class I molecules to CD8(+) T cells. We previously demonstrated that, when cell-associated ovalbumin (OVA) is injected into mice, only the CD8(+) DC subset cross-presents OVA in the context of MHC class I. In contrast to this selectivity with cell-associated antigen, we show here that both DC subsets isolated from mice injected with OVA/anti-OVA immune complexes (OVA-IC) cross-present OVA to CD8(+) T cells. The use of immunoglobulin G Fc receptor (Fc(gamma)R) common gamma-chain-deficient mice revealed that the cross-presentation by CD8(-) DCs depended on the expression of gamma-chain-containing activating FcgammaRs, whereas cross-presentation by CD8(+) DCs was not reduced in gamma-chain-deficient mice. These results suggest that although CD8(+) DCs constitutively cross-present exogenous antigens in the context of MHC class I molecules, CD8(-) DCs only do so after activation, such as via ligation of Fc(gamma)Rs. Cross-presentation of immune complexes may play an important role in autoimmune diseases and the therapeutic effect of antitumor antibodies.
Figures

Similar articles
-
Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance.J Exp Med. 2002 Dec 16;196(12):1627-38. doi: 10.1084/jem.20021598. J Exp Med. 2002. PMID: 12486105 Free PMC article.
-
A novel role of complement factor C1q in augmenting the presentation of antigen captured in immune complexes to CD8+ T lymphocytes.J Immunol. 2007 Jun 15;178(12):7581-6. doi: 10.4049/jimmunol.178.12.7581. J Immunol. 2007. PMID: 17548593
-
TLR7 triggering with polyuridylic acid promotes cross-presentation in CD8α+ conventional dendritic cells by enhancing antigen preservation and MHC class I antigen permanence on the dendritic cell surface.J Immunol. 2013 Feb 1;190(3):948-60. doi: 10.4049/jimmunol.1102725. Epub 2013 Jan 2. J Immunol. 2013. PMID: 23284054
-
[Development of effective antigen delivery carrier to dendritic cells via Fc receptor in cancer immunotherapy].Yakugaku Zasshi. 2007 Feb;127(2):301-6. doi: 10.1248/yakushi.127.301. Yakugaku Zasshi. 2007. PMID: 17268150 Review. Japanese.
-
Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens.Immunol Lett. 2007 Dec 15;114(2):66-72. doi: 10.1016/j.imlet.2007.09.007. Epub 2007 Oct 12. Immunol Lett. 2007. PMID: 17964665 Review.
Cited by
-
Modulating T Cell Responses via Autophagy: The Intrinsic Influence Controlling the Function of Both Antigen-Presenting Cells and T Cells.Front Immunol. 2018 Dec 14;9:2914. doi: 10.3389/fimmu.2018.02914. eCollection 2018. Front Immunol. 2018. PMID: 30619278 Free PMC article. Review.
-
Different routes of MHC-I delivery to phagosomes and their consequences to CD8 T cell immunity.Semin Immunol. 2023 Mar;66:101713. doi: 10.1016/j.smim.2023.101713. Epub 2023 Jan 25. Semin Immunol. 2023. PMID: 36706521 Free PMC article. Review.
-
Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza.Nat Immunol. 2010 Mar;11(3):216-24. doi: 10.1038/ni.1838. Epub 2010 Jan 24. Nat Immunol. 2010. PMID: 20098442 Free PMC article.
-
Dendritic cells and regulatory T cells expressing CCR4 provide resistance to coxsackievirus B5-induced pancreatitis.Sci Rep. 2019 Oct 14;9(1):14766. doi: 10.1038/s41598-019-51311-9. Sci Rep. 2019. PMID: 31611578 Free PMC article.
-
Coregulatory interactions among CD8α dendritic cells, the latency-associated transcript, and programmed death 1 contribute to higher levels of herpes simplex virus 1 latency.J Virol. 2014 Jun;88(12):6599-610. doi: 10.1128/JVI.00590-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672046 Free PMC article.
References
-
- Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. - PubMed
-
- Bevan, M.J. 1976. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 117:2233–2238. - PubMed
-
- Huang, A.Y., P. Golumbek, M. Ahmadzadeh, E. Jaffee, D. Pardoll, and H. Levitsky. 1994. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 264:961–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

