Craniosynostosis in transgenic mice overexpressing Nell-1
- PMID: 12235118
- PMCID: PMC151127
- DOI: 10.1172/JCI15375
Craniosynostosis in transgenic mice overexpressing Nell-1
Erratum in
- J Clin Invest 2002 Nov;110(10):1573
Abstract
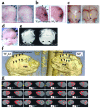
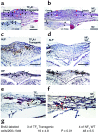
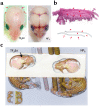
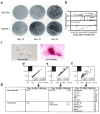
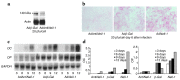
Previously, we reported NELL-1 as a novel molecule overexpressed during premature cranial suture closure in patients with craniosynostosis (CS), one of the most common congenital craniofacial deformities. Here we describe the creation and analysis of transgenic mice overexpressing Nell-1. Nell-1 transgenic animals exhibited CS-like phenotypes that ranged from simple to compound synostoses. Histologically, the osteogenic fronts of abnormally closing/closed sutures in these animals revealed calvarial overgrowth and overlap along with increased osteoblast differentiation and reduced cell proliferation. Furthermore, anomalies were restricted to calvarial bone, despite generalized, non-tissue-specific overexpression of Nell-1. In vitro, Nell-1 overexpression accelerated calvarial osteoblast differentiation and mineralization under normal culture conditions. Moreover, Nell-1 overexpression in osteoblasts was sufficient to promote alkaline phosphatase expression and micronodule formation. Conversely, downregulation of Nell-1 inhibited osteoblast differentiation in vitro. In summary, Nell-1 overexpression induced calvarial overgrowth resulting in premature suture closure in a rodent model. Nell-1, therefore, has a novel role in CS development, perhaps as part of a complex chain of events resulting in premature suture closure. On a cellular level, Nell-1 expression may modulate and be both sufficient and required for osteoblast differentiation.
Figures


Similar articles
-
Nell-1-induced bone regeneration in calvarial defects.Am J Pathol. 2006 Sep;169(3):903-15. doi: 10.2353/ajpath.2006.051210. Am J Pathol. 2006. PMID: 16936265 Free PMC article.
-
Overexpression of Nell-1, a craniosynostosis-associated gene, induces apoptosis in osteoblasts during craniofacial development.J Bone Miner Res. 2003 Dec;18(12):2126-34. doi: 10.1359/jbmr.2003.18.12.2126. J Bone Miner Res. 2003. PMID: 14672347
-
Nell-1 induces acrania-like cranioskeletal deformities during mouse embryonic development.Lab Invest. 2006 Jul;86(7):633-44. doi: 10.1038/labinvest.3700430. Epub 2006 May 1. Lab Invest. 2006. PMID: 16652108
-
The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration.J Dent Res. 2010 Sep;89(9):865-78. doi: 10.1177/0022034510376401. Epub 2010 Jul 20. J Dent Res. 2010. PMID: 20647499 Free PMC article. Review.
-
Roles of FGFR2 and twist in human craniosynostosis: insights from genetic mutations in cranial osteoblasts.Front Oral Biol. 2008;12:144-159. doi: 10.1159/000115036. Front Oral Biol. 2008. PMID: 18391499 Review.
Cited by
-
Structure-function analysis of Nel, a thrombospondin-1-like glycoprotein involved in neural development and functions.J Biol Chem. 2012 Jan 27;287(5):3282-91. doi: 10.1074/jbc.M111.281485. Epub 2011 Dec 8. J Biol Chem. 2012. PMID: 22157752 Free PMC article.
-
Nell-1-induced bone regeneration in calvarial defects.Am J Pathol. 2006 Sep;169(3):903-15. doi: 10.2353/ajpath.2006.051210. Am J Pathol. 2006. PMID: 16936265 Free PMC article.
-
Nfatc2 is a primary response gene of Nell-1 regulating chondrogenesis in ATDC5 cells.J Bone Miner Res. 2011 Jun;26(6):1230-41. doi: 10.1002/jbmr.314. J Bone Miner Res. 2011. PMID: 21611965 Free PMC article.
-
The Nell-1 growth factor stimulates bone formation by purified human perivascular cells.Tissue Eng Part A. 2011 Oct;17(19-20):2497-509. doi: 10.1089/ten.TEA.2010.0705. Epub 2011 Jul 18. Tissue Eng Part A. 2011. PMID: 21615216 Free PMC article.
-
Efficient production and characterization of recombinant human NELL1 protein in human embryonic kidney 293-F cells.Mol Biotechnol. 2012 May;51(1):58-66. doi: 10.1007/s12033-011-9440-4. Mol Biotechnol. 2012. PMID: 21814724
References
-
- Cohen, M.M, Jr., and MacLean, R.E. 2000. Craniosynostosis: diagnosis, evaluation and management. 2nd edition. Oxford University Press. New York, New York, USA. 454 pp.
-
- Carlton MB, Colledge WH, Evans MJ. Crouzon-like craniofacial dysmorphology in the mouse is caused by an insertional mutation at the Fgf3/Fgf4 locus. Dev Dyn. 1998;212:242–249. - PubMed
-
- Jabs EW, et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell. 1993;75:443–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

