A requirement for replication in activation of the ATR-dependent DNA damage checkpoint
- PMID: 12231621
- PMCID: PMC187437
- DOI: 10.1101/gad.1013502
A requirement for replication in activation of the ATR-dependent DNA damage checkpoint
Abstract
Using the Xenopus egg extract system, we investigated the involvement of DNA replication in activation of the DNA damage checkpoint. We show here that DNA damage slows replication in a checkpoint-independent manner and is accompanied by replication-dependent recruitment of ATR and Rad1 to chromatin. We also find that the replication proteins RPA and Polalpha accumulate on chromatin following DNA damage. Finally, damage-induced Chk1 phosphorylation and checkpoint arrest are abrogated when replication is inhibited. These data indicate that replication is required for activation of the DNA damage checkpoint and suggest a unifying model for ATR activation by diverse lesions during S phase.
Figures

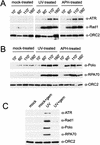
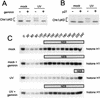
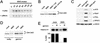
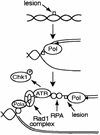
Similar articles
-
Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts.Genes Dev. 2000 Nov 1;14(21):2745-56. doi: 10.1101/gad.842500. Genes Dev. 2000. PMID: 11069891 Free PMC article.
-
Xenopus ATR is a replication-dependent chromatin-binding protein required for the DNA replication checkpoint.Curr Biol. 2000 Dec 14-28;10(24):1565-73. doi: 10.1016/s0960-9822(00)00855-1. Curr Biol. 2000. PMID: 11137007
-
Claspin, a Chk1-regulatory protein, monitors DNA replication on chromatin independently of RPA, ATR, and Rad17.Mol Cell. 2003 Feb;11(2):329-40. doi: 10.1016/s1097-2765(03)00045-5. Mol Cell. 2003. PMID: 12620222
-
Chk1 in the DNA damage response: conserved roles from yeasts to mammals.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1025-32. doi: 10.1016/j.dnarep.2004.03.003. DNA Repair (Amst). 2004. PMID: 15279789 Review.
-
Safeguarding genome integrity: the checkpoint kinases ATR, CHK1 and WEE1 restrain CDK activity during normal DNA replication.Nucleic Acids Res. 2012 Jan;40(2):477-86. doi: 10.1093/nar/gkr697. Epub 2011 Sep 21. Nucleic Acids Res. 2012. PMID: 21937510 Free PMC article. Review.
Cited by
-
An ATR- and BRCA1-mediated Fanconi anemia pathway is required for activating the G2/M checkpoint and DNA damage repair upon rereplication.Mol Cell Biol. 2006 Jun;26(12):4601-11. doi: 10.1128/MCB.02141-05. Mol Cell Biol. 2006. PMID: 16738325 Free PMC article.
-
Minichromosome maintenance proteins are direct targets of the ATM and ATR checkpoint kinases.Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10078-83. doi: 10.1073/pnas.0403410101. Epub 2004 Jun 21. Proc Natl Acad Sci U S A. 2004. PMID: 15210935 Free PMC article.
-
Molecular mechanisms of DNA replication checkpoint activation.Genes (Basel). 2014 Mar 6;5(1):147-75. doi: 10.3390/genes5010147. Genes (Basel). 2014. PMID: 24705291 Free PMC article.
-
MMB-FOXM1-driven premature mitosis is required for CHK1 inhibitor sensitivity.Cell Rep. 2021 Mar 2;34(9):108808. doi: 10.1016/j.celrep.2021.108808. Cell Rep. 2021. PMID: 33657372 Free PMC article.
-
Continued primer synthesis at stalled replication forks contributes to checkpoint activation.J Cell Biol. 2010 Apr 19;189(2):233-46. doi: 10.1083/jcb.200909105. Epub 2010 Apr 12. J Cell Biol. 2010. PMID: 20385778 Free PMC article.
References
-
- Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes & Dev. 2001;15:2177–2196. - PubMed
-
- Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: Partners in checkpoint signaling. Science. 2001;294:1713–1716. - PubMed
-
- D'Urso G, Grallert B, Nurse P. DNA polymerase α, a component of the replication initiation complex, is essential for the checkpoint coupling S phase to mitosis in fission yeast. J Cell Sci. 1995;108:3109–3118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
