Nuclear export and retention signals in the RS domain of SR proteins
- PMID: 12215544
- PMCID: PMC134038
- DOI: 10.1128/MCB.22.19.6871-6882.2002
Nuclear export and retention signals in the RS domain of SR proteins
Abstract
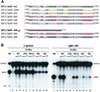
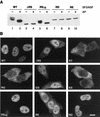
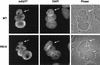
Splicing factors of the SR protein family share a modular structure consisting of one or two RNA recognition motifs (RRMs) and a C-terminal RS domain rich in arginine and serine residues. The RS domain, which is extensively phosphorylated, promotes protein-protein interactions and directs subcellular localization and-in certain situations-nucleocytoplasmic shuttling of individual SR proteins. We analyzed mutant versions of human SF2/ASF in which the natural RS repeats were replaced by RD or RE repeats and compared the splicing and subcellular localization properties of these proteins to those of SF2/ASF lacking the entire RS domain or possessing a minimal RS domain consisting of 10 consecutive RS dipeptides (RS10). In vitro splicing of a pre-mRNA that requires an RS domain could take place when the mutant RD, RE, or RS10 domain replaced the natural domain. The RS10 version of SF2/ASF shuttled between the nucleus and the cytoplasm in the same manner as the wild-type protein, suggesting that a tract of consecutive RS dipeptides, in conjunction with the RRMs of SF2/ASF, is necessary and sufficient to direct nucleocytoplasmic shuttling. However, the SR protein SC35 has two long stretches of RS repeats, yet it is not a shuttling protein. We demonstrate the presence of a dominant nuclear retention signal in the RS domain of SC35.
Figures
Similar articles
-
A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm.Genes Dev. 1998 Jan 1;12(1):55-66. doi: 10.1101/gad.12.1.55. Genes Dev. 1998. PMID: 9420331 Free PMC article.
-
Pre-mRNA splicing in the absence of an SR protein RS domain.Genes Dev. 2000 Dec 15;14(24):3166-78. doi: 10.1101/gad.189500. Genes Dev. 2000. PMID: 11124808 Free PMC article.
-
Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity.J Cell Biol. 1997 Jul 28;138(2):225-38. doi: 10.1083/jcb.138.2.225. J Cell Biol. 1997. PMID: 9230067 Free PMC article.
-
Deletion of the N-terminus of SF2/ASF permits RS-domain-independent pre-mRNA splicing.PLoS One. 2007 Sep 5;2(9):e854. doi: 10.1371/journal.pone.0000854. PLoS One. 2007. PMID: 17786225 Free PMC article.
-
Regiospecific phosphorylation control of the SR protein ASF/SF2 by SRPK1.J Mol Biol. 2009 Jul 24;390(4):618-34. doi: 10.1016/j.jmb.2009.05.060. Epub 2009 May 27. J Mol Biol. 2009. PMID: 19477182 Free PMC article.
Cited by
-
Splicing-factor oncoprotein SRSF1 stabilizes p53 via RPL5 and induces cellular senescence.Mol Cell. 2013 Apr 11;50(1):56-66. doi: 10.1016/j.molcel.2013.02.001. Epub 2013 Mar 7. Mol Cell. 2013. PMID: 23478443 Free PMC article.
-
The Ref/Aly proteins are dispensable for mRNA export and development in Caenorhabditis elegans.RNA. 2003 Jul;9(7):881-91. doi: 10.1261/rna.5420503. RNA. 2003. PMID: 12810921 Free PMC article.
-
Selective mutations in estrogen receptor alpha D-domain alters nuclear translocation and non-estrogen response element gene regulatory mechanisms.J Biol Chem. 2011 Apr 8;286(14):12640-9. doi: 10.1074/jbc.M110.187773. Epub 2011 Feb 1. J Biol Chem. 2011. PMID: 21285458 Free PMC article.
-
Regulation of human neurotropic JC virus replication by alternative splicing factor SF2/ASF in glial cells.PLoS One. 2011 Jan 31;6(1):e14630. doi: 10.1371/journal.pone.0014630. PLoS One. 2011. PMID: 21297941 Free PMC article.
-
Conserved arginines of bovine adenovirus-3 33K protein are important for transportin-3 mediated transport and virus replication.PLoS One. 2014 Jul 14;9(7):e101216. doi: 10.1371/journal.pone.0101216. eCollection 2014. PLoS One. 2014. PMID: 25019945 Free PMC article.
References
-
- Blencowe, B. J. 2000. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem. Sci. 25:106-110. - PubMed
-
- Cáceres, J. F., and A. R. Krainer. 1997. Mammalian pre-mRNA splicing factors, p. 174-212. In A. R. Krainer (ed.), Eukaryotic mRNA processing. IRL Press, Oxford, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
