Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis
- PMID: 12213723
- PMCID: PMC1867252
- DOI: 10.1016/S0002-9440(10)64255-1
Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis
Abstract
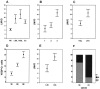
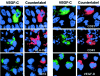
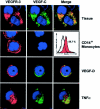
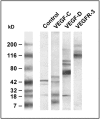
Formation of lymphatic metastasis is the initial step of generalized spreading of tumor cells and predicts poor clinical prognosis. Lymphatic vessels generally arise within the peritumoral stroma, although the lymphangiopoietic vascular endothelial growth factors (VEGF)-C and -D are produced by tumor cells. In a carefully selected collection of human cervical cancers (stage pT1b1) we demonstrate by quantitative immunohistochemistry and in situ hybridization that density of lymphatic microvessels is significantly increased in peritumoral stroma, and that a subset of stromal cells express large amounts of VEGF-C and VEGF-D. The density of cells producing these vascular growth factors correlates with peritumoral inflammatory stroma reaction, lymphatic microvessel density, and indirectly with peritumoral carcinomatous lymphangiosis and frequency of lymph node metastasis. The VEGF-C- and VEGF-D-producing stroma cells were identified in situ as a subset of activated tumor-associated macrophages (TAMs) by expression of a panel of macrophage-specific markers, including CD68, CD23, and CD14. These TAMs also expressed the VEGF-C- and VEGF-D-specific tyrosine kinase receptor VEGFR-3. As TAMs are derived from monocytes in the circulation, a search in peripheral blood for candidate precursors of VEGFR-3-expressing TAMs revealed a subfraction of CD14-positive, VEGFR-3-expressing monocytes, that, however, failed to express VEGF-C and VEGF-D. Only after in vitro incubation with tumor necrosis factor-alpha, lipopolysaccharide, or VEGF-D did these monocytes start to synthesize VEGF-C de novo. In conclusion VEGF-C-expressing TAMs play a novel role in peritumoral lymphangiogenesis and subsequent dissemination in human cancer.
Figures



Similar articles
-
Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium.J Rheumatol. 2002 Jan;29(1):39-45. J Rheumatol. 2002. PMID: 11824969
-
Semaphorin 4D expression is associated with a poor clinical outcome in cervical cancer patients.Microvasc Res. 2014 May;93:1-8. doi: 10.1016/j.mvr.2014.02.007. Epub 2014 Mar 3. Microvasc Res. 2014. PMID: 24603190
-
VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival.Surgery. 2006 Jun;139(6):839-46. doi: 10.1016/j.surg.2005.12.008. Surgery. 2006. PMID: 16782443
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
Lymphatic versus blood vascular endothelial growth factors and receptors in humans.Microsc Res Tech. 2001 Oct 15;55(2):108-21. doi: 10.1002/jemt.1162. Microsc Res Tech. 2001. PMID: 11596156 Review.
Cited by
-
Lymphatic vessels in health and disease.Wiley Interdiscip Rev Syst Biol Med. 2013 Jan-Feb;5(1):111-24. doi: 10.1002/wsbm.1201. Epub 2012 Dec 3. Wiley Interdiscip Rev Syst Biol Med. 2013. PMID: 23209022 Free PMC article. Review.
-
Dysregulation of Amphiregulin stimulates the pathogenesis of cystic lymphangioma.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2019580118. doi: 10.1073/pnas.2019580118. Proc Natl Acad Sci U S A. 2021. PMID: 33941693 Free PMC article.
-
The nuclear corepressor 1 and the thyroid hormone receptor β suppress breast tumor lymphangiogenesis.Oncotarget. 2016 Nov 29;7(48):78971-78984. doi: 10.18632/oncotarget.12978. Oncotarget. 2016. PMID: 27806339 Free PMC article.
-
Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy.Sci Rep. 2013;3:2375. doi: 10.1038/srep02375. Sci Rep. 2013. PMID: 23921465 Free PMC article.
-
Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages.J Clin Invest. 2005 Sep;115(9):2363-72. doi: 10.1172/JCI23874. J Clin Invest. 2005. PMID: 16138190 Free PMC article.
References
-
- Yamada Y, Nezu J, Shimane M, Hirata Y: Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics 1997, 42:483-488 - PubMed
-
- Partanen TA, Alitalo K, Miettinen M: Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer 1999, 86:2406-2412 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

