Assembly and translocation of papillomavirus capsid proteins
- PMID: 12208977
- PMCID: PMC136525
- DOI: 10.1128/jvi.76.19.10009-10014.2002
Assembly and translocation of papillomavirus capsid proteins
Abstract
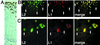
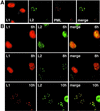
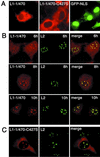
The major and minor capsid proteins of polyomavirus are preassembled in the cytoplasm and translocated to the nucleus only as a VP1-VP2/VP3 complex. In this study, we describe independent nuclear translocation of the L1 major protein and the L2 minor capsid protein of human papillomavirus type 33 by several approaches. First, we observed that expression and nuclear translocation of L2 in natural lesions precede expression of L1. Second, using a cell culture system for coexpression, we found that accumulation of L2 in nuclear domain 10 (ND10) subnuclear structures precedes L1 by several hours. In contrast, complexes of L2 and mutants of L1 forced to assemble in the cytoplasm are translocated directly to ND10, like L2 expressed alone. Interestingly, accumulation of wild-type L1 is observed only after L2-induced release of the ND10-associated protein Sp100. Third, nuclear translocation of L2 but not of L1 was blocked by the proteasome inhibitor MG132. Our data suggest that L1 and L2 interaction occurs after L2-induced reorganization of ND10 subnuclear domains.
Figures


Similar articles
-
Nuclear localization but not PML protein is required for incorporation of the papillomavirus minor capsid protein L2 into virus-like particles.J Virol. 2004 Feb;78(3):1121-8. doi: 10.1128/jvi.78.3.1121-1128.2004. J Virol. 2004. PMID: 14722267 Free PMC article.
-
Factors influencing subcellular localization of the human papillomavirus L2 minor structural protein.Virology. 2006 Feb 5;345(1):199-208. doi: 10.1016/j.virol.2005.09.047. Epub 2005 Oct 27. Virology. 2006. PMID: 16257028
-
Reorganization of nuclear domain 10 induced by papillomavirus capsid protein l2.Virology. 2002 Mar 30;295(1):97-107. doi: 10.1006/viro.2002.1360. Virology. 2002. PMID: 12033769
-
L2, the minor capsid protein of papillomavirus.Virology. 2013 Oct;445(1-2):175-86. doi: 10.1016/j.virol.2013.04.017. Epub 2013 May 17. Virology. 2013. PMID: 23689062 Free PMC article. Review.
-
Insights into the role and function of L2, the minor capsid protein of papillomaviruses.Arch Virol. 2009;154(2):187-97. doi: 10.1007/s00705-009-0310-3. Epub 2009 Jan 25. Arch Virol. 2009. PMID: 19169853 Review.
Cited by
-
Viruses and Skin Cancer.Int J Mol Sci. 2021 May 20;22(10):5399. doi: 10.3390/ijms22105399. Int J Mol Sci. 2021. PMID: 34065594 Free PMC article. Review.
-
Viruses in the Nucleus.Cold Spring Harb Perspect Biol. 2021 Aug 2;13(8):a039446. doi: 10.1101/cshperspect.a039446. Cold Spring Harb Perspect Biol. 2021. PMID: 33753405 Free PMC article. Review.
-
Involvement of Nucleophosmin (NPM1/B23) in Assembly of Infectious HPV16 Capsids.Papillomavirus Res. 2015 Dec;1:74-89. doi: 10.1016/j.pvr.2015.06.005. Epub 2015 Jun 25. Papillomavirus Res. 2015. PMID: 27398412 Free PMC article.
-
Nuclear translocation of papillomavirus minor capsid protein L2 requires Hsc70.J Virol. 2004 Jun;78(11):5546-53. doi: 10.1128/JVI.78.11.5546-5553.2004. J Virol. 2004. PMID: 15140951 Free PMC article.
-
Cruising the cellular highways: How human papillomavirus travels from the surface to the nucleus.Virus Res. 2017 Mar 2;231:1-9. doi: 10.1016/j.virusres.2016.10.015. Epub 2016 Oct 29. Virus Res. 2017. PMID: 27984059 Free PMC article. Review.
References
-
- Delos, S. E., L. Montross, R. B. Moreland, and R. L. Garcea. 1993. Expression of the polyoma VP2 and VP3 proteins in insect cells: coexpression with the major capsid protein VP1 alters VP2/3 localization. Virology 194:393-398. - PubMed
-
- Florin, L., F. Schäfer, K. Sotlar, R. E. Streeck, and M. Sapp. 2002. Reorganization of nuclear domain 10 induced by papillomavirus capsid protein L2. Virology 295:97-107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

