The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1
- PMID: 12208882
- PMCID: PMC2194005
- DOI: 10.1084/jem.20020267
The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1
Abstract
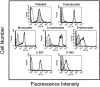
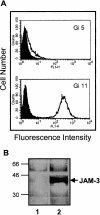
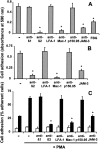
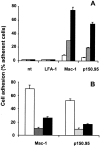
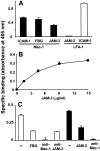
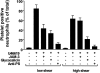
The recently described junctional adhesion molecules (JAMs) in man and mice are involved in homotypic and heterotypic intercellular interactions. Here, a third member of this family, human JAM-3, was identified and described as a novel counterreceptor on platelets for the leukocyte beta2-integrin Mac-1 (alphaMbeta2, CD11b/CD18). With the help of two monoclonal antibodies, Gi11 and Gi13, against a 43-kD surface glycoprotein on human platelets, a full-length cDNA encoding JAM-3 was identified. JAM-3 is a type I transmembrane glycoprotein containing two Ig-like domains. Although JAM-3 did not undergo homophilic interactions, myelo-monocytic cells adhered to immobilized JAM-3 or to JAM-3-transfected cells. This heterophilic interaction was specifically attributed to a direct interaction of JAM-3 with the beta2-integrin Mac-1 and to a lower extent with p150.95 (alphaXbeta2, CD11c/CD18) but not with LFA-1 (alphaLbeta2, CD11a/CD18) or with beta1-integrins. These results were corroborated by analysis of K562 erythroleukemic cells transfected with different heterodimeric beta2-integrins and by using purified proteins. Moreover, purified JAM-3 or antibodies against JAM-3 blocked the platelet-neutrophil interaction, indicating that platelet JAM-3 serves as a counterreceptor for Mac-1 mediating leukocyte-platelet interactions. JAM-3 thereby provides a novel molecular target for antagonizing interactions between vascular cells that promote inflammatory vascular pathologies such as in atherothrombosis.
Figures




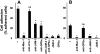
Similar articles
-
High molecular weight kininogen regulates platelet-leukocyte interactions by bridging Mac-1 and glycoprotein Ib.J Biol Chem. 2003 Nov 14;278(46):45375-81. doi: 10.1074/jbc.M304344200. Epub 2003 Sep 2. J Biol Chem. 2003. PMID: 12952972
-
Expression of junctional adhesion molecule-C on the surface of platelets supports adhesion, but not differentiation, of human CD34 cells in vitro.Cell Physiol Biochem. 2012;29(1-2):153-62. doi: 10.1159/000337596. Epub 2012 Mar 1. Cell Physiol Biochem. 2012. PMID: 22415084
-
The homophilic binding of junctional adhesion molecule-C mediates tumor cell-endothelial cell interactions.J Biol Chem. 2005 Oct 28;280(43):36326-33. doi: 10.1074/jbc.M505059200. Epub 2005 Aug 23. J Biol Chem. 2005. PMID: 16118203
-
Junctional adhesion molecule 1 (JAM-1).J Biol Regul Homeost Agents. 2003 Oct-Dec;17(4):341-7. J Biol Regul Homeost Agents. 2003. PMID: 15065765 Review.
-
The JAM family of junctional adhesion molecules.Curr Opin Cell Biol. 2003 Oct;15(5):525-30. doi: 10.1016/s0955-0674(03)00104-2. Curr Opin Cell Biol. 2003. PMID: 14519386 Review.
Cited by
-
Ligand recognition specificity of leukocyte integrin αMβ2 (Mac-1, CD11b/CD18) and its functional consequences.Biochemistry. 2015 Feb 17;54(6):1408-20. doi: 10.1021/bi5013782. Epub 2015 Feb 5. Biochemistry. 2015. PMID: 25613106 Free PMC article.
-
Pathophysiological mechanisms in acute pancreatitis: Current understanding.Indian J Gastroenterol. 2016 May;35(3):153-66. doi: 10.1007/s12664-016-0647-y. Epub 2016 May 21. Indian J Gastroenterol. 2016. PMID: 27206712 Review.
-
Neutrophil interactions with T cells, platelets, endothelial cells, and of course tumor cells.Immunol Rev. 2023 Mar;314(1):13-35. doi: 10.1111/imr.13178. Epub 2022 Dec 16. Immunol Rev. 2023. PMID: 36527200 Free PMC article. Review.
-
Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha.J Exp Med. 2003 Oct 6;198(7):1077-88. doi: 10.1084/jem.20022181. J Exp Med. 2003. PMID: 14530377 Free PMC article.
-
Interactions between Platelets and Tumor Microenvironment Components in Ovarian Cancer and Their Implications for Treatment and Clinical Outcomes.Cancers (Basel). 2023 Feb 17;15(4):1282. doi: 10.3390/cancers15041282. Cancers (Basel). 2023. PMID: 36831623 Free PMC article. Review.
References
-
- Springer, T.A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: a multistep paradigm. Cell. 76:301–314. - PubMed
-
- Languino, L.R., A. Duperray, K.J. Joganic, M. Fornaro, G.B. Thornton, and D.C. Altieri. 1995. Regulation of leukocyte-endothelial interactions and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc. Natl. Acad. Sci. USA. 92:7734–7738. - PMC - PubMed
-
- Marcus, A.J. 1994. Thrombosis and inflammation as multicellular processes: significance of cell-cell interactions. Semin. Hematol. 31:261–269. - PubMed
-
- Rinder, C.S., J.L. Bonan, H.M. Rinder, J. Matthew, R. Hines, and B.R. Smith. 1992. Cardiopulmonary bypass induces leukocyte-platelet adhesion. Blood. 79:1201–1205. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

