Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events
- PMID: 12196576
- PMCID: PMC2792199
- DOI: 10.1523/JNEUROSCI.22-17-07526.2002
Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events
Abstract
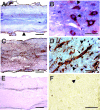
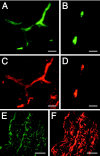
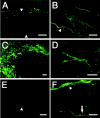
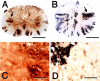
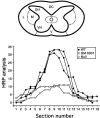
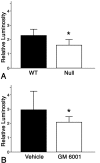
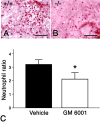
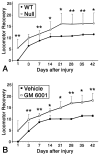
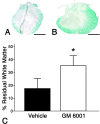
Inflammation in general and proteinases generated as a result are likely mediators of early secondary pathogenesis after spinal cord injury. We report that matrix metalloproteinase-9 (MMP-9) plays an important role in blood-spinal cord barrier dysfunction, inflammation, and locomotor recovery. MMP-9 was present in the meninges and neurons of the uninjured cord. MMP-9 increased rapidly after a moderate contusion spinal cord injury, reaching a maximum at 24 hr, becoming markedly reduced by 72 hr, and not detectable at 7 d after injury. It was seen in glia, macrophages, neutrophils, and vascular elements in the injured spinal cord at 24 hr after injury. The natural tissue inhibitors of MMPs were unchanged over this time course. MMP-9-null mice exhibited significantly less disruption of the blood-spinal cord barrier, attenuation of neutrophil infiltration, and significant locomotor recovery compared with wild-type mice. Similar findings were observed in mice treated with a hydroxamic acid MMP inhibitor from 3 hr to 3 d after injury, compared with the vehicle controls. Moreover, the area of residual white matter at the lesion epicenter was significantly greater in the inhibitor-treated group. This study provides evidence that MMP-9 plays a key role in abnormal vascular permeability and inflammation within the first 3 d after spinal cord injury, and that blockade of MMPs during this critical period attenuates these vascular events and leads to improved locomotor recovery. Our findings suggest that early inhibition of MMPs may be an efficacious strategy for the spinal cord-injured patient.
Figures

Similar articles
-
Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury.J Neurosci. 2006 Sep 27;26(39):9841-50. doi: 10.1523/JNEUROSCI.1993-06.2006. J Neurosci. 2006. PMID: 17005848 Free PMC article.
-
Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury.Am J Pathol. 2014 Nov;184(11):2985-3000. doi: 10.1016/j.ajpath.2014.07.016. Epub 2014 Oct 14. Am J Pathol. 2014. PMID: 25325922
-
Matrix metalloproteinases and their inhibitors in human traumatic spinal cord injury.BMC Neurol. 2007 Jun 26;7:17. doi: 10.1186/1471-2377-7-17. BMC Neurol. 2007. PMID: 17594482 Free PMC article.
-
Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury.Neurotherapeutics. 2011 Apr;8(2):206-20. doi: 10.1007/s13311-011-0038-0. Neurotherapeutics. 2011. PMID: 21455784 Free PMC article. Review.
-
Propitious Therapeutic Modulators to Prevent Blood-Spinal Cord Barrier Disruption in Spinal Cord Injury.Mol Neurobiol. 2017 Jul;54(5):3578-3590. doi: 10.1007/s12035-016-9910-6. Epub 2016 May 18. Mol Neurobiol. 2017. PMID: 27194298 Review.
Cited by
-
Extra Cellular Matrix Remodeling: An Adjunctive Target for Spinal Cord Injury and Intervertebral Disc Degeneration.Neurospine. 2022 Sep;19(3):632-645. doi: 10.14245/ns.2244366.183. Epub 2022 Sep 30. Neurospine. 2022. PMID: 36203290 Free PMC article.
-
Ethanol Extract of Bupleurum falcatum Improves Functional Recovery by Inhibiting Matrix Metalloproteinases-2 and -9 Activation and Inflammation after Spinal Cord Injury.Exp Neurobiol. 2010 Dec;19(3):146-54. doi: 10.5607/en.2010.19.3.146. Epub 2010 Dec 31. Exp Neurobiol. 2010. PMID: 22110354 Free PMC article.
-
Inflammatory Regulation of CNS Barriers After Traumatic Brain Injury: A Tale Directed by Interleukin-1.Front Immunol. 2021 May 21;12:688254. doi: 10.3389/fimmu.2021.688254. eCollection 2021. Front Immunol. 2021. PMID: 34093593 Free PMC article. Review.
-
Deficiency in matrix metalloproteinase-2 results in long-term vascular instability and regression in the injured mouse spinal cord.Exp Neurol. 2016 Oct;284(Pt A):50-62. doi: 10.1016/j.expneurol.2016.07.018. Epub 2016 Jul 25. Exp Neurol. 2016. PMID: 27468657 Free PMC article.
-
The optimal transplantation strategy of umbilical cord mesenchymal stem cells in spinal cord injury: a systematic review and network meta-analysis based on animal studies.Stem Cell Res Ther. 2022 Sep 2;13(1):441. doi: 10.1186/s13287-022-03103-8. Stem Cell Res Ther. 2022. PMID: 36056386 Free PMC article. Review.
References
-
- Adler RR, Brenner CA, Werb Z. Expression of extracellular matrix-degrading metalloproteinases and metalloproteinase inhibitors is developmentally regulated during endoderm differentiation of embryonal carcinoma cells. Development. 1990;110:211–220. - PubMed
-
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. - PubMed
-
- Armao D, Kornfeld M, Estrada EY, Grossetete M, Rosenberg GA. Neutral proteases and disruption of the blood-brain barrier in rat. Brain Res. 1997;767:259–264. - PubMed
-
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
