PPAR alpha is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue
- PMID: 12195019
- PMCID: PMC129357
- DOI: 10.1073/pnas.182420899
PPAR alpha is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue
Abstract
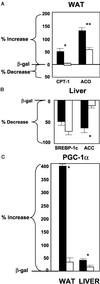
Adenovirus-induced hyperleptinemia causes rapid disappearance of body fat in normal rats, presumably by up-regulating fatty acid oxidation within white adipocytes. To determine the role of peroxisomal proliferation-activated receptor (PPAR)alpha expression, which was increased during the rapid loss of fat, we infused adenovirus-leptin into PPAR alpha(-/-) and PPAR alpha(+/+) mice. Despite similar degrees of hyperleptinemia and reduction in food intake, epididymal fat pad weight declined 55% in wild-type but only 6% in PPAR alpha(-/-) mice; liver triacylglycerol fell 39% in the wild-type group but was unchanged in PPAR(-/-) mice. Carnitine palmitoyl transferase-1 mRNA rose 52% in the wild-type mice but did not increase in PPAR alpha(-/-) mice. PPAR gamma coactivator-1 alpha rose 3-fold in the fat and 46% in the liver of wild-type mice but was unchanged in PPAR alpha(-/-) mice. Although AMP-activated protein kinase could not be implicated in the lipopenic actions of hyperleptinemia, acetyl CoA carboxylase protein was reduced in the liver of wild-type but not in PPAR alpha(-/-) mice. Thus, in PPAR alpha(-/-) mice, up-regulation of carnitine palmitoyl transferase-1 mRNA in fat, down-regulation of acetyl CoA carboxylase in liver, and up-regulation of PPAR gamma coactivator-1 alpha mRNA in both tissues are abolished, as is the reduction in their triacylglycerol content.
Figures





Similar articles
-
The role of leptin resistance in the lipid abnormalities of aging.FASEB J. 2001 Jan;15(1):108-114. doi: 10.1096/fj.00-0310com. FASEB J. 2001. PMID: 11149898
-
Differential gene regulation in human versus rodent hepatocytes by peroxisome proliferator-activated receptor (PPAR) alpha. PPAR alpha fails to induce peroxisome proliferation-associated genes in human cells independently of the level of receptor expresson.J Biol Chem. 2001 Aug 24;276(34):31521-7. doi: 10.1074/jbc.M103306200. Epub 2001 Jun 19. J Biol Chem. 2001. PMID: 11418601
-
Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha.J Biol Chem. 2004 Aug 13;279(33):35017-24. doi: 10.1074/jbc.M405327200. Epub 2004 Jun 4. J Biol Chem. 2004. PMID: 15180999
-
The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation.Biochim Biophys Acta. 1996 Jul 26;1302(2):93-109. doi: 10.1016/0005-2760(96)00066-5. Biochim Biophys Acta. 1996. PMID: 8695669 Review.
-
Regulation of the peroxisomal beta-oxidation-dependent pathway by peroxisome proliferator-activated receptor alpha and kinases.Biochem Pharmacol. 2000 Oct 15;60(8):1027-32. doi: 10.1016/s0006-2952(00)00416-0. Biochem Pharmacol. 2000. PMID: 11007938 Review.
Cited by
-
Astragalus mongholicus powder, a traditional Chinese medicine formula ameliorate type 2 diabetes by regulating adipoinsular axis in diabetic mice.Front Pharmacol. 2022 Aug 15;13:973927. doi: 10.3389/fphar.2022.973927. eCollection 2022. Front Pharmacol. 2022. PMID: 36046814 Free PMC article.
-
Leptin's role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals.Endocr Rev. 2013 Jun;34(3):377-412. doi: 10.1210/er.2012-1053. Epub 2013 Mar 8. Endocr Rev. 2013. PMID: 23475416 Free PMC article. Review.
-
Tissue factor-protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation.Nat Med. 2011 Oct 23;17(11):1490-7. doi: 10.1038/nm.2461. Nat Med. 2011. PMID: 22019885 Free PMC article.
-
Pathophysiology of NAFLD and NASH in Experimental Models: The Role of Food Intake Regulating Peptides.Front Endocrinol (Lausanne). 2020 Nov 26;11:597583. doi: 10.3389/fendo.2020.597583. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33324348 Free PMC article. Review.
-
Mitochondrial role in the neonatal predisposition to developing nonalcoholic fatty liver disease.J Clin Invest. 2018 Aug 31;128(9):3692-3703. doi: 10.1172/JCI120846. Epub 2018 Aug 31. J Clin Invest. 2018. PMID: 30168806 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

