Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins
- PMID: 12186918
- PMCID: PMC136451
- DOI: 10.1128/jvi.76.18.9355-9367.2002
Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins
Abstract
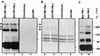
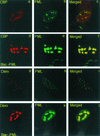
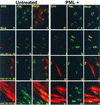
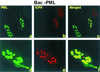
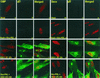
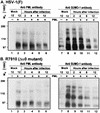
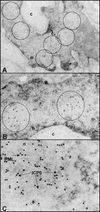
A key early event in the replication of herpes simplex virus 1 (HSV-1) is the localization of infected-cell protein no. 0 (ICP0) in nuclear structures knows as ND10 or promyelocytic leukemia oncogenic domains (PODs). This is followed by dispersal of ND10 constituents such as the promyelocytic leukemia protein (PML), CREB-binding protein (CBP), and Daxx. Numerous experiments have shown that this dispersal is mediated by ICP0. PML is thought to be the organizing structural component of ND10. To determine whether the virus targets PML because it is inimical to viral replication, telomerase-immortalized human foreskin fibroblasts and HEp-2 cells were transduced with wild-type baculovirus or a baculovirus expressing the M(r) 69,000 form of PML. The transduced cultures were examined for expression and localization of PML in mock-infected and HSV-1-infected cells. The results obtained from studies of cells overexpressing PML were as follows. (i) Transduced cells accumulate large amounts of unmodified and SUMO-I-modified PML. (ii) Mock-infected cells exhibited enlarged ND10 structures containing CBP and Daxx in addition to PML. (iii) In infected cells, ICP0 colocalized with PML in ND10 early in infection, but the two proteins did not overlap or were juxtaposed in orderly structures. (iv) The enlarged ND10 structures remained intact at least until 12 h after infection and retained CBP and Daxx in addition to PML. (v) Overexpression of PML had no effect on the accumulation of viral proteins representative of alpha, beta, or gamma groups and had no effect on the accumulation of infectious virus in cells infected with wild-type virus or a mutant (R7910) from which the alpha 0 genes had been deleted. These results indicate the following: (i) PML overexpressed in transduced cells cannot be differentiated from endogenous PML with respect to sumoylation and localization in ND10 structures. (ii) PML does not affect viral replication or the changes in the localization of ICP0 through infection. (iii) Disaggregation of ND10 structures is not an obligatory event essential for viral replication.
Figures

Similar articles
-
Recruitment of herpes simplex virus type 1 transcriptional regulatory protein ICP4 into foci juxtaposed to ND10 in live, infected cells.J Virol. 2003 Mar;77(6):3680-9. doi: 10.1128/jvi.77.6.3680-3689.2003. J Virol. 2003. PMID: 12610143 Free PMC article.
-
Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects.J Virol. 2003 Jun;77(12):7101-5. doi: 10.1128/jvi.77.12.7101-7105.2003. J Virol. 2003. PMID: 12768029 Free PMC article.
-
Visualization by live-cell microscopy of disruption of ND10 during herpes simplex virus type 1 infection.J Virol. 2004 Oct;78(20):11411-5. doi: 10.1128/JVI.78.20.11411-11415.2004. J Virol. 2004. PMID: 15452264 Free PMC article.
-
The use of fluorescence microscopy to study the association between herpesviruses and intrinsic resistance factors.Viruses. 2011 Dec;3(12):2412-24. doi: 10.3390/v3122412. Epub 2011 Dec 7. Viruses. 2011. PMID: 22355446 Free PMC article. Review.
-
Review: properties and assembly mechanisms of ND10, PML bodies, or PODs.J Struct Biol. 2000 Apr;129(2-3):278-87. doi: 10.1006/jsbi.2000.4239. J Struct Biol. 2000. PMID: 10806078 Review.
Cited by
-
Simian TRIM5alpha proteins reduce replication of herpes simplex virus.Virology. 2010 Mar 15;398(2):243-50. doi: 10.1016/j.virol.2009.11.041. Epub 2010 Jan 12. Virology. 2010. PMID: 20060996 Free PMC article.
-
Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against varicella-zoster virus.PLoS Pathog. 2011 Feb 3;7(2):e1001266. doi: 10.1371/journal.ppat.1001266. PLoS Pathog. 2011. PMID: 21304940 Free PMC article.
-
PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0.J Virol. 2003 Aug;77(16):8686-94. doi: 10.1128/jvi.77.16.8686-8694.2003. J Virol. 2003. PMID: 12885887 Free PMC article.
-
ICP0 antagonizes ICP4-dependent silencing of the herpes simplex virus ICP0 gene.PLoS One. 2010 Jan 21;5(1):e8837. doi: 10.1371/journal.pone.0008837. PLoS One. 2010. PMID: 20098619 Free PMC article.
-
PML isoforms I and II participate in PML-dependent restriction of HSV-1 replication.J Cell Sci. 2011 Jan 15;124(Pt 2):280-91. doi: 10.1242/jcs.075390. Epub 2010 Dec 20. J Cell Sci. 2011. PMID: 21172801 Free PMC article.
References
-
- Ahn, J. H., E. J. Brignole III, and G. S. Hayward. 1998. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 18:4899-4913. - PMC - PubMed
-
- Borden, K. L. 1998. RING fingers and B-boxes: zinc-binding protein-protein interaction domains. Biochem. Cell Biol. 76:351-358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

