The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting
- PMID: 12186843
- PMCID: PMC2196052
- DOI: 10.1084/jem.20011608
The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting
Erratum in
- J Exp Med 2002 Sep 16;196(6):869
Abstract
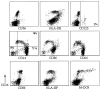
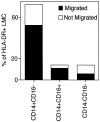
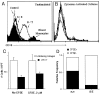
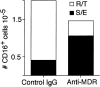
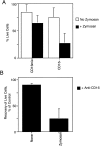
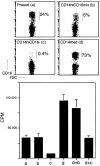
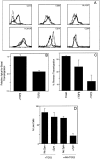
Much remains to be learned about the physiologic events that promote monocytes to become lymph-homing dendritic cells (DCs). In a model of transendothelial trafficking, some monocytes become DCs in response to endogenous signals. These DCs migrate across endothelium in the ablumenal-to-lumenal direction (reverse transmigration), reminiscent of the migration into lymphatic vessels. Here we show that the subpopulation of monocytes that expresses CD16 (Fcgamma receptor III) is predisposed to become migratory DCs. The vast majority of cells derived from CD16(+) monocytes reverse transmigrated, and their presence was associated with migratory cells expressing high levels of CD86 and human histocompatibility leukocyte antigen (HLA)-DR, and robust capacity to induce allogeneic T cell proliferation. A minority of CD16(-) monocytes reverse transmigrated, and these cells stimulated T cell proliferation less efficiently. CD16 was not functionally required for reverse transmigration, but promoted cell survival when yeast particles (zymosan) were present as a maturation stimulus in the subendothelial matrix. The cell surface phenotype and migratory characteristics of CD16(+) monocytes were inducible in CD16(-) monocytes by preincubation with TGFbeta1. We propose that CD16(+) monocytes may contribute significantly to precursors for DCs that transiently survey tissues and migrate to lymph nodes via afferent lymphatic vessels.
Figures

Similar articles
-
Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking.Science. 1998 Oct 16;282(5388):480-3. doi: 10.1126/science.282.5388.480. Science. 1998. PMID: 9774276
-
CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics.Eur J Immunol. 2000 Jul;30(7):1872-83. doi: 10.1002/1521-4141(200007)30:7<1872::AID-IMMU1872>3.0.CO;2-2. Eur J Immunol. 2000. PMID: 10940876
-
Induction of suppressive phenotype in monocyte-derived dendritic cells by leukemic cell products and IL-1β.Hum Immunol. 2014 Jul;75(7):641-9. doi: 10.1016/j.humimm.2014.04.013. Epub 2014 Apr 24. Hum Immunol. 2014. PMID: 24768898
-
Myeloid DCs deduced from monocytes. In-vitro and in-vivo data support a monocytic origin of DCs.Adv Exp Med Biol. 1997;417:27-32. Adv Exp Med Biol. 1997. PMID: 9286333 Review. No abstract available.
-
Factors and signals that govern the migration of dendritic cells via lymphatics: recent advances.Springer Semin Immunopathol. 2005 Jan;26(3):273-87. doi: 10.1007/s00281-004-0168-0. Springer Semin Immunopathol. 2005. PMID: 15338191 Review.
Cited by
-
The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting.Annu Rev Immunol. 2013;31:563-604. doi: 10.1146/annurev-immunol-020711-074950. Annu Rev Immunol. 2013. PMID: 23516985 Free PMC article. Review.
-
Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques.J Clin Invest. 2007 Jan;117(1):185-94. doi: 10.1172/JCI28549. J Clin Invest. 2007. PMID: 17200718 Free PMC article.
-
CD1a expression defines an interleukin-12 producing population of human dendritic cells.Clin Exp Immunol. 2009 Mar;155(3):523-33. doi: 10.1111/j.1365-2249.2008.03853.x. Clin Exp Immunol. 2009. PMID: 19220838 Free PMC article.
-
Intermediate monocytes contribute to pathologic immune response in Leishmania braziliensis infections.J Infect Dis. 2015 Jan 15;211(2):274-82. doi: 10.1093/infdis/jiu439. Epub 2014 Aug 19. J Infect Dis. 2015. PMID: 25139016 Free PMC article.
-
Treated and Untreated Primary Progressive Multiple Sclerosis: Walkthrough Immunological Changes of Monocytes and T Regulatory Cells.Biomedicines. 2024 Feb 19;12(2):464. doi: 10.3390/biomedicines12020464. Biomedicines. 2024. PMID: 38398067 Free PMC article.
References
-
- O'Doherty, U., R.M. Steinman, M. Peng, P.U. Cameron, S. Gezelter, I. Kopeloff, W.J. Swiggard, M. Pope, and N. Bhardwaj. 1993. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J. Exp. Med. 178:1067–1076. - PMC - PubMed
-
- Thomas, R., L.S. Davis, and P.E. Lipsky. 1993. Isolation and characterization of human peripheral blood dendritic cells. J. Immunol. 150:821–834. - PubMed
-
- Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. - PubMed
-
- Randolph, G.J., S. Beaulieu, S. Lebecque, R.M. Steinman, and W.A. Muller. 1998. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 282:480–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

