DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response
- PMID: 12183369
- PMCID: PMC186436
- DOI: 10.1101/gad.1008902
DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response
Abstract
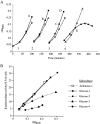
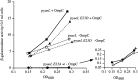
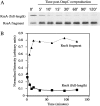
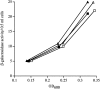
All cells have stress response pathways that maintain homeostasis in each cellular compartment. In the Gram-negative bacterium Escherichia coli, the sigma(E) pathway responds to protein misfolding in the envelope. The stress signal is transduced across the inner membrane to the cytoplasm via the inner membrane protein RseA, the anti-sigma factor that inhibits the transcriptional activity of sigma(E). Stress-induced activation of the pathway requires the regulated proteolysis of RseA. In this report we show that RseA is degraded by sequential proteolytic events controlled by the inner membrane-anchored protease DegS and the membrane-embedded metalloprotease YaeL, an ortholog of mammalian Site-2 protease (S2P). This is consistent with the mechanism of activation of ATF6, the mammalian unfolded protein response transcription factor by Site-1 protease and S2P. Thus, mammalian and bacterial cells employ a conserved proteolytic mechanism to activate membrane-associated transcription factors that initiate intercompartmental cellular stress responses.
Figures



Similar articles
-
YaeL (EcfE) activates the sigma(E) pathway of stress response through a site-2 cleavage of anti-sigma(E), RseA.Genes Dev. 2002 Aug 15;16(16):2147-55. doi: 10.1101/gad.1002302. Genes Dev. 2002. PMID: 12183368 Free PMC article.
-
Fine-tuning of the Escherichia coli sigmaE envelope stress response relies on multiple mechanisms to inhibit signal-independent proteolysis of the transmembrane anti-sigma factor, RseA.Genes Dev. 2004 Nov 1;18(21):2686-97. doi: 10.1101/gad.1238604. Genes Dev. 2004. PMID: 15520285 Free PMC article.
-
The Escherichia coli sigma(E)-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-sigma factor.Genes Dev. 1999 Sep 15;13(18):2449-61. doi: 10.1101/gad.13.18.2449. Genes Dev. 1999. PMID: 10500101 Free PMC article.
-
Regulated proteolysis: control of the Escherichia coli σ(E)-dependent cell envelope stress response.Subcell Biochem. 2013;66:129-60. doi: 10.1007/978-94-007-5940-4_6. Subcell Biochem. 2013. PMID: 23479440 Review.
-
Regulation of the Escherichia coli sigma-dependent envelope stress response.Mol Microbiol. 2004 May;52(3):613-9. doi: 10.1111/j.1365-2958.2003.03982.x. Mol Microbiol. 2004. PMID: 15101969 Review.
Cited by
-
Two stress sensor proteins for the expression of sigmaE regulon: DegS and RseB.J Microbiol. 2015 May;53(5):306-10. doi: 10.1007/s12275-015-5112-6. Epub 2015 May 3. J Microbiol. 2015. PMID: 25935301 Review.
-
The activity of σV, an extracytoplasmic function σ factor of Bacillus subtilis, is controlled by regulated proteolysis of the anti-σ factor RsiV.J Bacteriol. 2013 Jul;195(14):3135-44. doi: 10.1128/JB.00292-13. Epub 2013 May 17. J Bacteriol. 2013. PMID: 23687273 Free PMC article.
-
Short-tailed stx phages exploit the conserved YaeT protein to disseminate Shiga toxin genes among enterobacteria.J Bacteriol. 2007 Oct;189(20):7223-33. doi: 10.1128/JB.00824-07. Epub 2007 Aug 10. J Bacteriol. 2007. PMID: 17693515 Free PMC article.
-
A protease and a lipoprotein jointly modulate the conserved ExoR-ExoS-ChvI signaling pathway critical in Sinorhizobium meliloti for symbiosis with legume hosts.PLoS Genet. 2023 Oct 23;19(10):e1010776. doi: 10.1371/journal.pgen.1010776. eCollection 2023 Oct. PLoS Genet. 2023. PMID: 37871041 Free PMC article.
-
Coregulation of gene expression by sigma factors RpoE and RpoS in Salmonella enterica serovar Typhi during hyperosmotic stress.Curr Microbiol. 2011 May;62(5):1483-9. doi: 10.1007/s00284-011-9890-8. Epub 2011 Feb 11. Curr Microbiol. 2011. PMID: 21311887
References
-
- Alba BM, Zhong HJ, Pelayo JC, Gross CA. degS (hhoB) is an essential Escherichia coli gene whose indispensable function is to provide σEactivity. Mol Microbiol. 2001;40:1323–1333. - PubMed
-
- Bartolomé B, Jubete Y, Martinez E, de la Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. - PubMed
-
- Beebe KD, Shin J, Peng J, Chaudhury C, Khera J, Pei D. Substrate recognition through a PDZ domain in tail-specific protease. Biochemistry. 2000;39:3149–3155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
