Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and protein substrates of proteasome
- PMID: 12181345
- PMCID: PMC117941
- DOI: 10.1091/mbc.e02-03-0122
Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and protein substrates of proteasome
Abstract
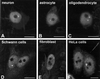
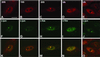
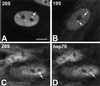
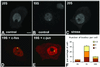
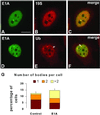
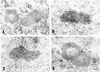
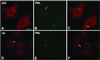
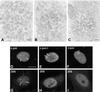
Nuclear bodies represent a heterogeneous class of nuclear structures. Herein, we describe that a subset of nuclear bodies is highly enriched in components of the ubiquitin-proteasome pathway of proteolysis. We coined the term clastosome (from the Greek klastos, broken and soma, body) to refer to this type of nuclear body. Clastosomes contain a high concentration of 1) ubiquitin conjugates, 2) the proteolytically active 20S core and the 19S regulatory complexes of the 26S proteasome, and 3) protein substrates of the proteasome. Although detected in a variety of cell types, clastosomes are scarce under normal conditions; however, they become more abundant when proteasomal activity is stimulated. In contrast, clastosomes disappear when cells are treated with proteasome inhibitors. Protein substrates of the proteasome that are found concentrated in clastosomes include the short-lived transcription factors c-Fos and c-Jun, adenovirus E1A proteins, and the PML protein. We propose that clastosomes are sites where proteolysis of a variety of protein substrates is taking place.
Figures

Similar articles
-
Promyelocytic leukemia protein is redistributed during the formation of intranuclear inclusions independent of polyglutamine expansion: an immunohistochemical study on Marinesco bodies.J Neuropathol Exp Neurol. 2002 Nov;61(11):984-91. doi: 10.1093/jnen/61.11.984. J Neuropathol Exp Neurol. 2002. PMID: 12430715
-
p14ARF induces the relocation of HDM2 and p53 to extranucleolar sites that are targeted by PML bodies and proteasomes.Mol Cancer. 2003 Mar 5;2:18. doi: 10.1186/1476-4598-2-18. Mol Cancer. 2003. PMID: 12685933 Free PMC article.
-
Proteasome-dependent processing of nuclear proteins is correlated with their subnuclear localization.J Struct Biol. 2002 Oct-Dec;140(1-3):189-99. doi: 10.1016/s1047-8477(02)00527-0. J Struct Biol. 2002. PMID: 12490167
-
Substrate access and processing by the 20S proteasome core particle.Int J Biochem Cell Biol. 2003 May;35(5):606-16. doi: 10.1016/s1357-2725(02)00390-4. Int J Biochem Cell Biol. 2003. PMID: 12672453 Review.
-
PML and COP1--two proteins with much in common.Trends Biochem Sci. 2001 Jan;26(1):18-20. doi: 10.1016/s0968-0004(00)01732-1. Trends Biochem Sci. 2001. PMID: 11165511 Review.
Cited by
-
A nervous system-specific subnuclear organelle in Caenorhabditis elegans.Genetics. 2021 Mar 3;217(1):1-17. doi: 10.1093/genetics/iyaa016. Genetics. 2021. PMID: 33683371 Free PMC article.
-
Inhibitory effect of ubiquitin-proteasome pathway on proliferation of esophageal carcinoma cells.World J Gastroenterol. 2004 Oct 1;10(19):2779-84. doi: 10.3748/wjg.v10.i19.2779. World J Gastroenterol. 2004. PMID: 15334669 Free PMC article.
-
Interaction of U-box E3 ligase SNEV with PSMB4, the beta7 subunit of the 20 S proteasome.Biochem J. 2005 Jun 1;388(Pt 2):593-603. doi: 10.1042/BJ20041517. Biochem J. 2005. PMID: 15660529 Free PMC article.
-
The Spatial Organization of the Intranuclear Structures of Human Brain Dopaminergic Neurons.Acta Naturae. 2017 Jul-Sep;9(3):81-88. Acta Naturae. 2017. PMID: 29104779 Free PMC article.
-
Apoptin nucleocytoplasmic shuttling is required for cell type-specific localization, apoptosis, and recruitment of the anaphase-promoting complex/cyclosome to PML bodies.J Virol. 2006 Aug;80(15):7535-45. doi: 10.1128/JVI.02741-05. J Virol. 2006. PMID: 16840333 Free PMC article.
References
-
- Arribas J, Arizti P, Castaño JG. Antibodies against the C2 COOH-terminal region discriminate the active and latent forms of the multicatalytic proteinase complex. J Biol Chem. 1994;269:12858–12864. - PubMed
-
- Baumeister W, Walz J, Zühl F, Seemüller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:3673–3680. - PubMed
-
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1467. - PubMed
-
- Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem. 1997;272:9002–9010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

