Potent VEGF blockade causes regression of coopted vessels in a model of neuroblastoma
- PMID: 12177446
- PMCID: PMC123268
- DOI: 10.1073/pnas.172398399
Potent VEGF blockade causes regression of coopted vessels in a model of neuroblastoma
Abstract
Vascular endothelial growth factor (VEGF) plays a key role in human tumor angiogenesis. We compared the effects of inhibitors of VEGF with different specificities in a xenograft model of neuroblastoma. Cultured human neuroblastoma NGP-GFP cells were implanted intrarenally in nude mice. Three anti-VEGF agents were tested: an anti-human VEGF(165) RNA-based fluoropyrimidine aptamer; a monoclonal anti-human VEGF antibody; and VEGF-Trap, a composite decoy receptor based on VEGFR-1 and VEGFR-2 fused to an Fc segment of IgG1. A wide range of efficacy was observed, with high-dose VEGF-Trap causing the greatest inhibition of tumor growth (81% compared with controls). We examined tumor angiogenesis and found that early in tumor formation, cooption of host vasculature occurs. We postulate that this coopted vasculature serves as a source of blood supply during the initial phase of tumor growth. Subsequently, control tumors undergo vigorous growth and remodeling of vascular networks, which results in disappearance of the coopted vessels. However, if VEGF function is blocked, cooption of host vessels may persist. Persistent cooption, therefore, may represent a novel mechanism by which neuroblastoma can partly evade antiangiogenic therapy and may explain why experimental neuroblastoma is less susceptible to VEGF blockade than a parallel model of Wilms tumor. However, more effective VEGF blockade, as achieved by high doses of VEGF-Trap, can lead to regression of coopted vascular structures. These results demonstrate that cooption of host vasculature is an early event in tumor formation, and that persistence of this effect is related to the degree of blockade of VEGF activity.
Figures
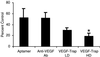



Similar articles
-
Distinct response of experimental neuroblastoma to combination antiangiogenic strategies.J Pediatr Surg. 2002 Mar;37(3):518-22. doi: 10.1053/jpsu.2002.30855. J Pediatr Surg. 2002. PMID: 11877680
-
Anti-VEGF antibody in experimental hepatoblastoma: suppression of tumor growth and altered angiogenesis.J Pediatr Surg. 2003 Mar;38(3):308-14; discussion 308-14. doi: 10.1053/jpsu.2003.50099. J Pediatr Surg. 2003. PMID: 12632340
-
All angiogenesis is not the same: Distinct patterns of response to antiangiogenic therapy in experimental neuroblastoma and Wilms tumor.J Pediatr Surg. 2001 Feb;36(2):287-90. doi: 10.1053/jpsu.2001.20691. J Pediatr Surg. 2001. PMID: 11172417
-
Tumor angiogenesis and accessibility: role of vascular endothelial growth factor.Semin Oncol. 2002 Dec;29(6 Suppl 16):3-9. doi: 10.1053/sonc.2002.37265. Semin Oncol. 2002. PMID: 12516032 Review.
-
'Accidental' anti-angiogenic drugs. anti-oncogene directed signal transduction inhibitors and conventional chemotherapeutic agents as examples.Eur J Cancer. 2000 Jun;36(10):1248-57. doi: 10.1016/s0959-8049(00)00092-7. Eur J Cancer. 2000. PMID: 10882863 Review.
Cited by
-
Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor.Oncologist. 2015 Jun;20(6):660-73. doi: 10.1634/theoncologist.2014-0465. Epub 2015 May 22. Oncologist. 2015. PMID: 26001391 Free PMC article. Review.
-
Controlling escape from angiogenesis inhibitors.Nat Rev Cancer. 2012 Oct;12(10):699-709. doi: 10.1038/nrc3366. Nat Rev Cancer. 2012. PMID: 23001349 Free PMC article. Review.
-
HAUSP deubiquitinates and stabilizes N-Myc in neuroblastoma.Nat Med. 2016 Oct;22(10):1180-1186. doi: 10.1038/nm.4180. Epub 2016 Sep 12. Nat Med. 2016. PMID: 27618649 Free PMC article.
-
Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer.J Clin Oncol. 2014 Aug 10;32(23):2463-70. doi: 10.1200/JCO.2013.51.4109. Epub 2014 Jul 7. J Clin Oncol. 2014. PMID: 25002722 Free PMC article. Clinical Trial.
-
Revisiting tumor angiogenesis: vessel co-option, vessel remodeling, and cancer cell-derived vasculature formation.Chin J Cancer. 2016 Jan 8;35:10. doi: 10.1186/s40880-015-0070-2. Chin J Cancer. 2016. PMID: 26747273 Free PMC article. Review.
References
-
- Leung D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. (1989) Science 246, 1306-1309. - PubMed
-
- Ferrara N. & Alitalo, K. (1999) Nat. Med. 5, 1359-1364. - PubMed
-
- Rowe D. H., Huang, J., Li, J., Manley, C., O'Toole, K. M., Stolar, C. J., Yamashiro, D. J. & Kandel, J. J. (2000) J. Pediatr. Surg. 35, 977-981. - PubMed
-
- Rowe D. H., Huang, J., Kayton, M. L., Thompson, R., Troxel, A., O'Toole, K. M., Yamashiro, D., Stolar, C. J. & Kandel, J. J. (2000) J. Pediatr. Surg. 35, 30-32. - PubMed
-
- Holash J., Wiegand, S. J. & Yancopoulos, G. D. (1999) Oncogene 18, 5356-5362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

