Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes
- PMID: 12163566
- PMCID: PMC2193942
- DOI: 10.1084/jem.20020110
Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes
Abstract
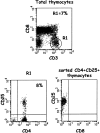
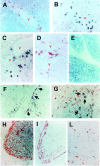
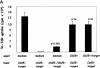
Phenotypic markers, localization, functional activities, and mechanisms of action in vitro of CD4(+)CD25(+) T cells, purified from postnatal human thymuses, were investigated. These cells showed poor or no proliferation in mixed lymphocyte culture (MLC), and suppressed in a dose-dependent fashion the proliferative response to allogeneic stimulation of CD4(+)CD25(-) thymocytes. Virtually all CD4(+)CD25(+) thymocytes constitutively expressed cytoplasmic T lymphocyte antigen (CTLA)-4, surface tumor necrosis factor type 2 receptor (TNFR2), and CCR8. They prevalently localized to perivascular areas of fibrous septa and responded to the chemoattractant activity of CCL1/I-309, which was found to be produced by either thymic medullary macrophages or fibrous septa epithelial cells. After polyclonal activation, CD4(+)CD25(+) thymocytes did not produce the cytokines interleukin (IL)-2, IL-4, IL-5, IL-13, interferon gamma, and only a very few produced IL-10, but all they expressed on their surface CTLA-4 and the majority of them also transforming growth factor (TGF)-beta1. The suppressive activity of these cells was contact dependent and associated with the lack of IL-2 receptor (IL-2R) alpha-chain (CD25) expression in target cells. Such a suppressive activity was partially inhibited by either anti-CTLA-4 or anti-TGF-beta1, and was completely blocked by a mixture of these monoclonal antibodies, which were also able to restore in target T cells the expression of IL-2R alpha-chain and, therefore, their responsiveness to IL-2. These data demonstrate that CD4(+)CD25(+) human thymocytes represent a population of regulatory cells that migrate in response to the chemokine CCL1/I-309 and exert their suppressive function via the inhibition of IL-2R alpha-chain in target T cells, induced by the combined activity of CTLA-4 and membrane TGF-beta1.
Figures


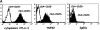
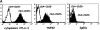
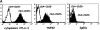






Similar articles
-
Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes.Blood. 2003 Dec 1;102(12):4107-14. doi: 10.1182/blood-2003-04-1320. Epub 2003 Jul 31. Blood. 2003. PMID: 12893750
-
Human CD4+CD25+ T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production.J Allergy Clin Immunol. 2003 Apr;111(4):862-8. doi: 10.1067/mai.2003.1412. J Allergy Clin Immunol. 2003. PMID: 12704370
-
Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function.J Exp Med. 2001 Jun 4;193(11):1295-302. doi: 10.1084/jem.193.11.1295. J Exp Med. 2001. PMID: 11390436 Free PMC article.
-
Thymic regulatory T cells.Autoimmun Rev. 2005 Nov;4(8):579-86. doi: 10.1016/j.autrev.2005.04.010. Autoimmun Rev. 2005. PMID: 16214099 Review.
-
The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets.J Leukoc Biol. 2003 Oct;74(4):471-8. doi: 10.1189/jlb.0503228. J Leukoc Biol. 2003. PMID: 14519757 Free PMC article. Review.
Cited by
-
Distinctive Leukocyte Subpopulations According to Organ Type in Cynomolgus Macaques.Comp Med. 2016;66(4):308-23. Comp Med. 2016. PMID: 27538862 Free PMC article.
-
Suppressor T cells in human diseases.J Exp Med. 2004 Aug 2;200(3):273-6. doi: 10.1084/jem.20040812. Epub 2004 Jul 26. J Exp Med. 2004. PMID: 15280423 Free PMC article.
-
Regulatory T-cell trafficking: from thymic development to tumor-induced immune suppression.Crit Rev Immunol. 2010;30(5):435-47. doi: 10.1615/critrevimmunol.v30.i5.30. Crit Rev Immunol. 2010. PMID: 21083525 Free PMC article. Review.
-
Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade.PLoS Pathog. 2009 Feb;5(2):e1000313. doi: 10.1371/journal.ppat.1000313. Epub 2009 Feb 27. PLoS Pathog. 2009. PMID: 19247441 Free PMC article.
-
CCR5 is required for regulation of alloreactive T-cell responses to single class II MHC-mismatched murine cardiac grafts.Am J Transplant. 2009 Oct;9(10):2251-61. doi: 10.1111/j.1600-6143.2009.02786.x. Epub 2009 Jul 28. Am J Transplant. 2009. PMID: 19656127 Free PMC article.
References
-
- Sakaguchi, S. 2000. Regulatory T cell: key controllers of immunologic self-tolerance. Cell. 101:455–458. - PubMed
-
- Shevach, E.M. 2000. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 18:423–449. - PubMed
-
- Suri-Payer, E., A.Z. Amar, R. McHugh, K. Natarajan, D.H. Margulies, and E.M. Shevach. 1999. Postéthymectomy autoimmune gastritis: fine specificity and pathogenicity of anti-H/K ATPase reactive T cells. Eur. J. Immunol. 29:669–677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

