Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis
- PMID: 12145198
- PMCID: PMC126150
- DOI: 10.1093/emboj/cdf404
Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis
Abstract
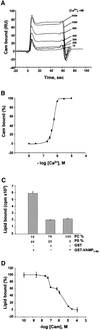
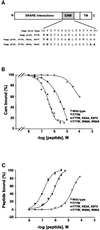
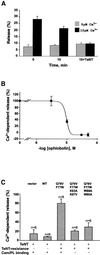
Neurotransmitter release involves the assembly of a heterotrimeric SNARE complex composed of the vesicle protein synaptobrevin (VAMP 2) and two plasma membrane partners, syntaxin 1 and SNAP-25. Calcium influx is thought to control this process via Ca(2+)-binding proteins that associate with components of the SNARE complex. Ca(2+)/calmodulin or phospholipids bind in a mutually exclusive fashion to a C-terminal domain of VAMP (VAMP(77-90)), and residues involved were identified by plasmon resonance spectroscopy. Microinjection of wild-type VAMP(77-90), but not mutant peptides, inhibited catecholamine release from chromaffin cells monitored by carbon fibre amperometry. Pre-incubation of PC12 pheochromocytoma cells with the irreversible calmodulin antagonist ophiobolin A inhibited Ca(2+)-dependent human growth hormone release in a permeabilized cell assay. Treatment of permeabilized cells with tetanus toxin light chain (TeNT) also suppressed secretion. In the presence of TeNT, exocytosis was restored by transfection of TeNT-resistant (Q(76)V, F(77)W) VAMP, but additional targeted mutations in VAMP(77-90) abolished its ability to rescue release. The calmodulin- and phospholipid-binding domain of VAMP 2 is thus required for Ca(2+)-dependent exocytosis, possibly to regulate SNARE complex assembly.
Figures



Similar articles
-
Calmodulin-dependent regulation of a lipid binding domain in the v-SNARE synaptobrevin and its role in vesicular fusion.Biol Cell. 2003 Oct;95(7):459-64. doi: 10.1016/s0248-4900(03)00076-5. Biol Cell. 2003. PMID: 14597264 Review.
-
Ca2+-dependent regulation of synaptic SNARE complex assembly via a calmodulin- and phospholipid-binding domain of synaptobrevin.Proc Natl Acad Sci U S A. 2000 Aug 15;97(17):9695-700. doi: 10.1073/pnas.97.17.9695. Proc Natl Acad Sci U S A. 2000. PMID: 10944231 Free PMC article.
-
A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells.Mol Biol Cell. 1998 Jun;9(6):1437-48. doi: 10.1091/mbc.9.6.1437. Mol Biol Cell. 1998. PMID: 9614185 Free PMC article.
-
Evidence for SNARE zippering during Ca2+-triggered exocytosis in PC12 cells.Neuropharmacology. 2003 Nov;45(6):777-86. doi: 10.1016/s0028-3908(03)00318-6. Neuropharmacology. 2003. PMID: 14529716
-
The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane: fast and slow pathways?Traffic. 2005 May;6(5):366-73. doi: 10.1111/j.1600-0854.2005.00288.x. Traffic. 2005. PMID: 15813747 Review.
Cited by
-
Characterization of P4 ATPase Phospholipid Translocases (Flippases) in Human and Rat Pancreatic Beta Cells: THEIR GENE SILENCING INHIBITS INSULIN SECRETION.J Biol Chem. 2015 Sep 18;290(38):23110-23. doi: 10.1074/jbc.M115.655027. Epub 2015 Aug 3. J Biol Chem. 2015. PMID: 26240149 Free PMC article.
-
Evidence that electrostatic interactions between vesicle-associated membrane protein 2 and acidic phospholipids may modulate the fusion of transport vesicles with the plasma membrane.Mol Biol Cell. 2009 Dec;20(23):4910-9. doi: 10.1091/mbc.e09-04-0284. Epub 2009 Oct 7. Mol Biol Cell. 2009. PMID: 19812247 Free PMC article.
-
Functional and genomic changes in the mouse ocular motor system in response to light deprivation from birth.J Neurosci. 2004 Jan 7;24(1):161-9. doi: 10.1523/JNEUROSCI.3234-03.2004. J Neurosci. 2004. PMID: 14715949 Free PMC article.
-
Structural domains involved in the regulation of transmitter release by synapsins.J Neurosci. 2005 Mar 9;25(10):2658-69. doi: 10.1523/JNEUROSCI.4278-04.2005. J Neurosci. 2005. PMID: 15758176 Free PMC article.
-
A common mechanism for the regulation of vesicular SNAREs on phospholipid membranes.Biochem J. 2004 Feb 1;377(Pt 3):781-5. doi: 10.1042/BJ20031164. Biochem J. 2004. PMID: 14563208 Free PMC article.
References
-
- Artalejo C.R., Elhamdani,A. and Palfrey,H.C. (1996) Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron, 16, 195–205. - PubMed
-
- Bittner M.A. and Holz,R.W. (1992) A temperature-sensitive step in exocytosis. J. Biol. Chem., 267, 16226–16229. - PubMed
-
- Chen Y.A., Duvvuri,V., Schulman,H. and Scheller,R.H. (1999a) Calmodulin and protein kinase C increase Ca2+-stimulated secretion by modulating membrane-attached exocytic machinery. J. Biol. Chem., 274, 26469–26476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

