Identification of a nuclear Stat1 protein tyrosine phosphatase
- PMID: 12138178
- PMCID: PMC133976
- DOI: 10.1128/MCB.22.16.5662-5668.2002
Identification of a nuclear Stat1 protein tyrosine phosphatase
Abstract
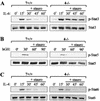
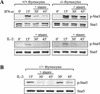
Upon interferon (IFN) stimulation, Stat1 becomes tyrosine phosphorylated and translocates into the nucleus, where it binds to DNA to activate transcription. The activity of Stat1 is dependent on tyrosine phosphorylation, and its inactivation in the nucleus is accomplished by a previously unknown protein tyrosine phosphatase (PTP). We have now purified a Stat1 PTP activity from HeLa cell nuclear extract and identified it as TC45, the nuclear isoform of the T-cell PTP (TC-PTP). TC45 can dephosphorylate Stat1 both in vitro and in vivo. Nuclear extracts lacking TC45 fail to dephosphorylate Stat1. Furthermore, the dephosphorylation of IFN-induced tyrosine-phosphorylated Stat1 is defective in TC-PTP-null mouse embryonic fibroblasts (MEFs) and primary thymocytes. Reconstitution of TC-PTP-null MEFs with TC45, but not the endoplasmic reticulum (ER)-associated isoform TC48, rescues the defect in Stat1 dephosphorylation. The dephosphorylation of Stat3, but not Stat5 or Stat6, is also affected in TC-PTP-null cells. Our results identify TC45 as a PTP responsible for the dephosphorylation of Stat1 in the nucleus.
Figures





Similar articles
-
The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation.Biochem Biophys Res Commun. 2002 Oct 4;297(4):811-7. doi: 10.1016/s0006-291x(02)02291-x. Biochem Biophys Res Commun. 2002. PMID: 12359225
-
Nuclear phosphatases and the proteasome in suppression of STAT1 activity in hepatocytes.Biochem Biophys Res Commun. 2002 Dec 13;299(4):574-80. doi: 10.1016/s0006-291x(02)02694-3. Biochem Biophys Res Commun. 2002. PMID: 12459177
-
The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase.EMBO J. 1996 Nov 15;15(22):6262-8. EMBO J. 1996. PMID: 8947049 Free PMC article.
-
A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus.Mol Endocrinol. 2002 Jan;16(1):58-69. doi: 10.1210/mend.16.1.0761. Mol Endocrinol. 2002. Retraction in: Mol Endocrinol. 2013 Nov;27(11):1982. doi: 10.1210/me.2013-1264 PMID: 11773439 Retracted.
-
The role of T-cell protein tyrosine phosphatase in epithelial carcinogenesis.Mol Carcinog. 2019 Sep;58(9):1640-1647. doi: 10.1002/mc.23078. Epub 2019 Jul 1. Mol Carcinog. 2019. PMID: 31264291 Free PMC article. Review.
Cited by
-
Strain-dependent differences in bone development, myeloid hyperplasia, morbidity and mortality in ptpn2-deficient mice.PLoS One. 2012;7(5):e36703. doi: 10.1371/journal.pone.0036703. Epub 2012 May 8. PLoS One. 2012. PMID: 22590589 Free PMC article.
-
Human β-defensin 3 induces STAT1 phosphorylation, tyrosine phosphatase activity, and cytokine synthesis in T cells.J Leukoc Biol. 2013 Sep;94(3):459-71. doi: 10.1189/jlb.0612300. Epub 2013 Jun 26. J Leukoc Biol. 2013. PMID: 23804808 Free PMC article.
-
Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2.Hepatology. 2009 Dec;50(6):1861-70. doi: 10.1002/hep.23214. Hepatology. 2009. PMID: 19821497 Free PMC article.
-
Protein Tyrosine Phosphatases as Potential Regulators of STAT3 Signaling.Int J Mol Sci. 2018 Sep 11;19(9):2708. doi: 10.3390/ijms19092708. Int J Mol Sci. 2018. PMID: 30208623 Free PMC article. Review.
-
The role of the inhibitors of interleukin-6 signal transduction SHP2 and SOCS3 for desensitization of interleukin-6 signalling.Biochem J. 2004 Mar 1;378(Pt 2):449-60. doi: 10.1042/BJ20030893. Biochem J. 2004. PMID: 14611646 Free PMC article.
References
-
- Aoki, N., and T. Matsuda. 2000. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J. Biol. Chem. 275:39718-39726. - PubMed
-
- Aoki, N., and T. Matsuda. 2002. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol. Endocrinol. 16:58-69. - PubMed
-
- Bowman, T., R. Garcia, J. Turkson, and R. Jove. 2000. STATs in oncogenesis. Oncogene 19:2474-2488. - PubMed
-
- Callus, B. A., and B. Mathey-Prevot. 1998. Interleukin-3-induced activation of the JAK/STAT pathway is prolonged by proteasome inhibitors. Blood 91:3182-3192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
