Identification of two additional translation products from the matrix (M) gene that contribute to vesicular stomatitis virus cytopathology
- PMID: 12134006
- PMCID: PMC155163
- DOI: 10.1128/jvi.76.16.8011-8018.2002
Identification of two additional translation products from the matrix (M) gene that contribute to vesicular stomatitis virus cytopathology
Abstract
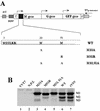
The matrix (M) protein of vesicular stomatitis virus (VSV) is a multifunctional protein that is responsible for condensation of the ribonucleocapsid core during virus assembly and also plays a critical role in virus budding. The M protein is also responsible for most of the cytopathic effects (CPE) observed in infected cells. VSV CPE include inhibition of host gene expression, disablement of nucleocytoplasmic transport, and disruption of the host cytoskeleton, which results in rounding of infected cells. In this report, we show that the VSV M gene codes for two additional polypeptides, which we have named M2 and M3. These proteins are synthesized from downstream methionines in the same open reading frame as the M protein (which we refer to here as M1) and lack the first 32 (M2) or 50 (M3) amino acids of M1. Infection of cells with a recombinant virus that does not express M2 and M3 (M33,51A) resulted in a delay in cell rounding, but virus yield was not affected. Transient expression of M2 and M3 alone caused cell rounding similar to that with the full-length M1 protein, suggesting that the cell-rounding function of the M protein does not require the N-terminal 50 amino acids. To determine if M2 and M3 were sufficient for VSV-mediated CPE, both M2 and M3 were expressed from a separate cistron in a VSV mutant background that readily establishes persistent infections and that normally lacks CPE. Infection of cells with the recombinant virus that expressed M2 and M3 resulted in cell rounding indistinguishable from that with the wild-type recombinant virus. These results suggest that M2 and M3 are important for cell rounding and may play an important role in viral cytopathogenesis. To our knowledge, this is first report of the multiple coding capacities of a rhabdovirus matrix gene.
Figures
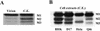




Similar articles
-
Impact of Vesicular Stomatitis Virus M Proteins on Different Cellular Functions.PLoS One. 2015 Jun 19;10(6):e0131137. doi: 10.1371/journal.pone.0131137. eCollection 2015. PLoS One. 2015. PMID: 26091335 Free PMC article.
-
Activity of vesicular stomatitis virus M protein mutants in cell rounding is correlated with the ability to inhibit host gene expression and is not correlated with virus assembly function.Virology. 1997 Mar 3;229(1):77-89. doi: 10.1006/viro.1996.8415. Virology. 1997. PMID: 9123880
-
Matrix protein of VSV New Jersey serotype containing methionine to arginine substitutions at positions 48 and 51 allows near-normal host cell gene expression.Virology. 2007 Jan 5;357(1):41-53. doi: 10.1016/j.virol.2006.07.022. Epub 2006 Sep 7. Virology. 2007. PMID: 16962155
-
Role of matrix protein in cytopathogenesis of vesicular stomatitis virus.J Virol. 1990 Apr;64(4):1716-25. doi: 10.1128/JVI.64.4.1716-1725.1990. J Virol. 1990. PMID: 2157054 Free PMC article.
-
Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus.J Virol. 2001 Dec;75(24):12169-81. doi: 10.1128/JVI.75.24.12169-12181.2001. J Virol. 2001. PMID: 11711608 Free PMC article.
Cited by
-
Rhabdovirus matrix protein structures reveal a novel mode of self-association.PLoS Pathog. 2008 Dec;4(12):e1000251. doi: 10.1371/journal.ppat.1000251. Epub 2008 Dec 26. PLoS Pathog. 2008. PMID: 19112510 Free PMC article.
-
A novel nuclear trafficking module regulates the nucleocytoplasmic localization of the rabies virus interferon antagonist, P protein.J Biol Chem. 2012 Aug 10;287(33):28112-21. doi: 10.1074/jbc.M112.374694. Epub 2012 Jun 14. J Biol Chem. 2012. PMID: 22700958 Free PMC article.
-
Rapid pathogenesis induced by a vesicular stomatitis virus matrix protein mutant: viral pathogenesis is linked to induction of tumor necrosis factor alpha.J Virol. 2006 Jul;80(14):7028-36. doi: 10.1128/JVI.00478-06. J Virol. 2006. PMID: 16809308 Free PMC article.
-
Identification of very small open reading frames in the genomes of Holmes Jungle virus, Ord River virus, and Wongabel virus of the genus Hapavirus, family Rhabdoviridae.Evol Bioinform Online. 2017 Jul 11;13:1176934317713484. doi: 10.1177/1176934317713484. eCollection 2017. Evol Bioinform Online. 2017. PMID: 28747815 Free PMC article. Review.
-
Interferon response and viral evasion by members of the family rhabdoviridae.Viruses. 2009 Dec;1(3):832-51. doi: 10.3390/v1030832. Epub 2009 Nov 9. Viruses. 2009. PMID: 21994572 Free PMC article.
References
-
- Cattaneo, R., K. Kaelin, K. Baczko, and M. A. Billeter. 1989. Measles virus editing provides an additional cysteine-rich protein. Cell 56:759-764. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

