Characterization of four types of background potassium channels in rat cerebellar granule neurons
- PMID: 12122143
- PMCID: PMC2290413
- DOI: 10.1113/jphysiol.2002.017590
Characterization of four types of background potassium channels in rat cerebellar granule neurons
Abstract
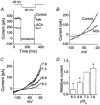
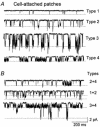
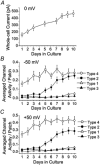
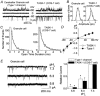
Cerebellar granule neurons express a standing outward (background) K+ current (I(K,SO)) that regulates the resting membrane potential and cell excitability. As several tandem-pore (2P) K+ channel mRNAs are highly expressed in cerebellar granule cells, we studied whether, and which, 2P K+ channels contribute to I(K,SO). I(K,SO) was highly sensitive to changes in extracellular pH and was partially inhibited by acetylcholine, as reported previously. In cell-attached patches from cultured cerebellar granule neurons, four types of K+ channels were found to be active when membrane potential was held at -50 mV or +50 mV in symmetrical 140 mM KCl. Based on single-channel conductances, gating kinetics and modulation by pharmacological agents and pH, three K+ channels could be considered as functional correlates of TASK-1, TASK-3 and TREK-2, which are members of the 2P K+ channel family. The fourth K+ channel (Type 4) has not been described previously and its molecular correlate is not yet known. Based on the measurement of channel current densities, the Type 2 (TASK-3) and the Type 4 K+ channels were determined to be the major sources of I(K,SO) in cultured cerebellar granule neurons. The Type 1 (TASK-1) and Type 3 (TREK-2) activities were relatively low throughout cell growth in culture (1-10 days). Similar to TASK-1 and TASK-3, the Type 4 K+ channel was highly sensitive to changes in extracellular pH, showing a 78 % inhibition by changing the extracellular pH from 7.3 to 6.3. The results of this study show that three 2P K+ channels and an additional pH-sensing K+ channel (Type 4) comprise the I(K,SO) in cultured cerebellar granule neurons. Our results also show that the high sensitivity of I(K,SO) to extracellular pH comes from the high sensitivity of Type 2 (TASK-3) and Type 4 K+ channels.
Figures



Comment in
-
Background potassium channels move into focus.J Physiol. 2002 Jul 15;542(Pt 2):334. doi: 10.1113/jphysiol.2002.020008. J Physiol. 2002. PMID: 12122133 Free PMC article. No abstract available.
Similar articles
-
Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels.EMBO J. 2003 Oct 15;22(20):5403-11. doi: 10.1093/emboj/cdg528. EMBO J. 2003. PMID: 14532113 Free PMC article.
-
Identification of native rat cerebellar granule cell currents due to background K channel KCNK5 (TASK-2).Brain Res Mol Brain Res. 2004 Sep 28;128(2):112-20. doi: 10.1016/j.molbrainres.2004.06.007. Brain Res Mol Brain Res. 2004. PMID: 15363886
-
Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells.J Physiol. 2004 Jan 1;554(Pt 1):64-77. doi: 10.1113/jphysiol.2003.054387. J Physiol. 2004. PMID: 14678492 Free PMC article.
-
What are the roles of the many different types of potassium channel expressed in cerebellar granule cells?Cerebellum. 2003;2(1):11-25. doi: 10.1080/14734220310015593. Cerebellum. 2003. PMID: 12882230 Review.
-
Pharmacology of neuronal background potassium channels.Neuropharmacology. 2003 Jan;44(1):1-7. doi: 10.1016/s0028-3908(02)00339-8. Neuropharmacology. 2003. PMID: 12559116 Review.
Cited by
-
Role of K₂p channels in stimulus-secretion coupling.Pflugers Arch. 2015 May;467(5):1001-11. doi: 10.1007/s00424-014-1663-3. Epub 2014 Dec 6. Pflugers Arch. 2015. PMID: 25476848 Free PMC article. Review.
-
Modulation of K2P3.1 (TASK-1), K2P9.1 (TASK-3), and TASK-1/3 heteromer by reactive oxygen species.Pflugers Arch. 2012 Nov;464(5):471-80. doi: 10.1007/s00424-012-1159-y. Epub 2012 Sep 25. Pflugers Arch. 2012. PMID: 23007462 Free PMC article.
-
Properties and modulation of the G protein-coupled K+ channel in rat cerebellar granule neurons: ATP versus phosphatidylinositol 4,5-bisphosphate.J Physiol. 2003 Aug 1;550(Pt 3):693-706. doi: 10.1113/jphysiol.2003.042119. Epub 2003 Jun 13. J Physiol. 2003. PMID: 12807991 Free PMC article.
-
Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels.EMBO J. 2003 Oct 15;22(20):5403-11. doi: 10.1093/emboj/cdg528. EMBO J. 2003. PMID: 14532113 Free PMC article.
-
Thyrotropin-releasing hormone increases GABA release in rat hippocampus.J Physiol. 2006 Dec 1;577(Pt 2):497-511. doi: 10.1113/jphysiol.2006.118141. Epub 2006 Sep 21. J Physiol. 2006. PMID: 16990402 Free PMC article.
References
-
- Ashmole I, Goodwin PA, Stanfield PR. TASK-5, a novel member of the tandem pore K+ channel family. Pflügers Archiv. 2001;442:828–833. - PubMed
-
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. Journal of Biological Chemistry. 2000;275:17412–17419. - PubMed
-
- Berberian G, Hidalgo C, Dipolo R, Beauge L. ATP stimulation of Na+/Ca2+ exchange in cardiac sarcolemmal vesicles. American Journal of Physiology. 1998;274:C724–733. - PubMed
-
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

