Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands
- PMID: 12097641
- PMCID: PMC126621
- DOI: 10.1073/pnas.142005099
Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands
Abstract
A number of poorly characterized genetic modifiers contribute to the extensive variability of von Willebrand disease, the most prevalent bleeding disorder in humans. We find that a genetic lesion inactivating the murine ST3Gal-IV sialyltransferase causes a bleeding disorder associated with an autosomal dominant reduction in plasma von Willebrand factor (VWF) and an autosomal recessive thrombocytopenia. Although both ST3Gal-IV and ST6Gal-I sialyltransferases mask galactose linkages implicated as asialoglycoprotein receptor ligands, only ST3Gal-IV deficiency promotes asialoglycoprotein clearance mechanisms with a reduction in plasma levels of VWF and platelets. Exposed galactose on VWF was also found in a subpopulation of humans with abnormally low VWF levels. Oligosaccharide branch-specific sialylation by the ST3Gal-IV sialyltransferase is required to sustain the physiologic half-life of murine hemostatic components and may be an important modifier of plasma VWF level in humans.
Figures
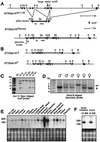
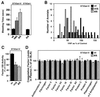
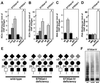
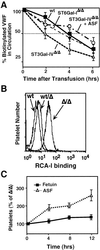
Similar articles
-
Role of sialic acid for platelet life span: exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes.Blood. 2009 Aug 20;114(8):1645-54. doi: 10.1182/blood-2009-01-199414. Epub 2009 Jun 11. Blood. 2009. PMID: 19520807 Free PMC article.
-
Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands.Blood. 2012 Aug 2;120(5):1015-26. doi: 10.1182/blood-2012-04-424366. Epub 2012 Jun 13. Blood. 2012. PMID: 22700726 Free PMC article.
-
Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in peripheral blood mononuclear cells in systemic sclerosis.J Rheumatol. 2004 Jan;31(1):88-95. J Rheumatol. 2004. PMID: 14705225
-
Physiological Roles of the von Willebrand Factor-Factor VIII Interaction.Subcell Biochem. 2020;94:437-464. doi: 10.1007/978-3-030-41769-7_18. Subcell Biochem. 2020. PMID: 32189311 Review.
-
Characterization of mouse sialyltransferase genes: their evolution and diversity.Biosci Biotechnol Biochem. 2008 May;72(5):1155-67. doi: 10.1271/bbb.80025. Epub 2008 May 7. Biosci Biotechnol Biochem. 2008. PMID: 18460788 Review.
Cited by
-
Biosynthesis of the major brain gangliosides GD1a and GT1b.Glycobiology. 2012 Oct;22(10):1289-301. doi: 10.1093/glycob/cws103. Epub 2012 Jun 26. Glycobiology. 2012. PMID: 22735313 Free PMC article.
-
Selectins and Immune Cells in Acute Myocardial Infarction and Post-infarction Ventricular Remodeling: Pathophysiology and Novel Treatments.Front Immunol. 2019 Feb 27;10:300. doi: 10.3389/fimmu.2019.00300. eCollection 2019. Front Immunol. 2019. PMID: 30873166 Free PMC article. Review.
-
The Ashwell receptor mitigates the lethal coagulopathy of sepsis.Nat Med. 2008 Jun;14(6):648-55. doi: 10.1038/nm1760. Epub 2008 May 18. Nat Med. 2008. PMID: 18488037 Free PMC article.
-
Innate mechanism of mucosal barrier erosion in the pathogenesis of acquired colitis.iScience. 2023 Sep 9;26(10):107883. doi: 10.1016/j.isci.2023.107883. eCollection 2023 Oct 20. iScience. 2023. PMID: 37752945 Free PMC article.
-
Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation.J Exp Med. 2008 Jun 9;205(6):1435-46. doi: 10.1084/jem.20070846. Epub 2008 Jun 2. J Exp Med. 2008. PMID: 18519646 Free PMC article.
References
-
- Ruggeri Z M, Ware J, Ginsberg D. In: Thrombosis and Hemorrhage. Loscalzo J, Schafer A I, editors. Baltimore: Williams & Wilkins; 1998. pp. 337–364.
-
- Sadler J E. Annu Rev Biochem. 1998;67:395–424. - PubMed
-
- Nichols W C, Ginsburg D. Medicine (Baltimore) 1997;76:1–20. - PubMed
-
- Berkowitz S D, Federici A B. Blood. 1988;72:1790–1796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

