Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles
- PMID: 12097581
- PMCID: PMC136371
- DOI: 10.1128/jvi.76.15.7672-7682.2002
Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles
Erratum in
- J Virol 2002 Sep;76(18):9562
Abstract
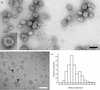
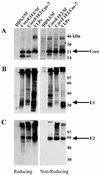
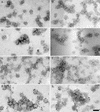
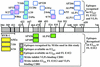
Purification of hepatitis C virus (HCV) from sera of infected patients has proven elusive, hampering efforts to perform structure-function analysis of the viral components. Recombinant forms of the viral glycoproteins have been used instead for functional studies, but uncertainty exists as to whether they closely mimic the virion proteins. Here, we used HCV virus-like particles (VLPs) generated in insect cells infected with a recombinant baculovirus expressing viral structural proteins. Electron microscopic analysis revealed a population of pleomorphic VLPs that were at least partially enveloped with bilayer membranes and had viral glycoprotein spikes protruding from the surface. Immunogold labeling using specific monoclonal antibodies (MAbs) demonstrated these protrusions to be the E1 and E2 glycoproteins. A panel of anti-E2 MAbs was used to probe the surface topology of E2 on the VLPs and to compare the antigenicity of the VLPs with that of truncated E2 (E2(660)) or the full-length (FL) E1E2 complex expressed in mammalian cells. While most MAbs bound to all forms of antigen, a number of others showed striking differences in their abilities to recognize the various E2 forms. All MAbs directed against hypervariable region 1 (HVR-1) recognized both native and denatured E2(660) with comparable affinities, but most bound either weakly or not at all to the FL E1E2 complex or to VLPs. HVR-1 on VLPs was accessible to these MAbs only after denaturation. Importantly, a subset of MAbs specific for amino acids 464 to 475 and 524 to 535 recognized E2(660) but not VLPs or FL E1E2 complex. The antigenic differences between E2(660,) FL E1E2, and VLPs strongly point to the existence of structural differences, which may have functional relevance. Trypsin treatment of VLPs removed the N-terminal part of E2, resulting in a 42-kDa fragment. In the presence of detergent, this was further reduced to a trypsin-resistant 25-kDa fragment, which could be useful for structural studies.
Figures


Similar articles
-
Functional analysis of hepatitis C virus E2 glycoproteins and virus-like particles reveals structural dissimilarities between different forms of E2.J Gen Virol. 2001 Aug;82(Pt 8):1877-1883. doi: 10.1099/0022-1317-82-8-1877. J Gen Virol. 2001. PMID: 11457993
-
Probing the antigenicity of hepatitis C virus envelope glycoprotein complex by high-throughput mutagenesis.PLoS Pathog. 2017 Dec 18;13(12):e1006735. doi: 10.1371/journal.ppat.1006735. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29253863 Free PMC article.
-
Immunizations with chimeric hepatitis B virus-like particles to induce potential anti-hepatitis C virus neutralizing antibodies.Antivir Ther. 2007;12(4):477-87. Antivir Ther. 2007. PMID: 17668556
-
Structural features of envelope proteins on hepatitis C virus-like particles as determined by anti-envelope monoclonal antibodies and CD81 binding.Virology. 2002 Jun 20;298(1):124-32. doi: 10.1006/viro.2002.1463. Virology. 2002. PMID: 12093180
-
Computational Modeling of Hepatitis C Virus Envelope Glycoprotein Structure and Recognition.Front Immunol. 2018 May 28;9:1117. doi: 10.3389/fimmu.2018.01117. eCollection 2018. Front Immunol. 2018. PMID: 29892287 Free PMC article. Review.
Cited by
-
Toward a hepatitis C virus vaccine: the structural basis of hepatitis C virus neutralization by AP33, a broadly neutralizing antibody.J Virol. 2012 Dec;86(23):12923-32. doi: 10.1128/JVI.02052-12. Epub 2012 Sep 19. J Virol. 2012. PMID: 22993159 Free PMC article.
-
Mouse Models for Studying HCV Vaccines and Therapeutic Antibodies.Methods Mol Biol. 2019;1911:481-503. doi: 10.1007/978-1-4939-8976-8_33. Methods Mol Biol. 2019. PMID: 30593647 Free PMC article.
-
Identification of a residue in hepatitis C virus E2 glycoprotein that determines scavenger receptor BI and CD81 receptor dependency and sensitivity to neutralizing antibodies.J Virol. 2008 Dec;82(24):12020-9. doi: 10.1128/JVI.01569-08. Epub 2008 Oct 1. J Virol. 2008. PMID: 18829747 Free PMC article.
-
Role of cleavage at the core-E1 junction of hepatitis C virus polyprotein in viral morphogenesis.PLoS One. 2017 Apr 24;12(4):e0175810. doi: 10.1371/journal.pone.0175810. eCollection 2017. PLoS One. 2017. PMID: 28437468 Free PMC article.
-
Generation and Characterization of Monoclonal Antibodies against a Cyclic Variant of Hepatitis C Virus E2 Epitope 412-422.J Virol. 2016 Jan 27;90(7):3745-59. doi: 10.1128/JVI.02397-15. J Virol. 2016. PMID: 26819303 Free PMC article.
References
-
- Al-Khayat, H. A., D. Bhella, J. M. Kenney, J. F. Roth, A. J. Kingsman, E. Martin-Rendon, and H. R. Saibil. 1999. Yeast Ty retrotransposons assemble into virus-like particles whose T-numbers depend on the C-terminal length of the capsid protein. J. Mol. Biol. 292:65-73. - PubMed
-
- Baumert, T. F., J. Vergalla, J. Satoi, M. Thomson, M. Lechmann, D. Herion, H. B. Greenberg, S. Ito, and T. J. Liang. 1999. Hepatitis C virus-like particles synthesized in insect cells as a potential vaccine candidate. Gastroenterology 117:1397-1407. - PubMed
-
- Bielefeldt Ohmann, H., and B. Bloch. 1982. Electron microscopic studies of bovine viral diarrhea virus in tissues of diseased calves and in cell cultures. Arch. Virol. 71:57-74. - PubMed
-
- Bishop, D. H. L. 1992. Baculovirus expression vectors. Semin. Virol. 3:253-264.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

