Experimental coinfection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: pathogenesis
- PMID: 12097580
- PMCID: PMC136401
- DOI: 10.1128/jvi.76.15.7661-7671.2002
Experimental coinfection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: pathogenesis
Abstract
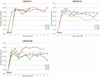
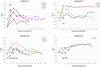
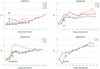
Human cytomegalovirus (HCMV) possesses low pathogenic potential in an immunocompetent host. In the immunosuppressed host, however, a wide spectrum of infection outcomes, ranging from asymptomatic to life threatening, can follow either primary or nonprimary infection. The variability in the manifestations of HCMV infection in immunosuppressed individuals implies that there is a threshold of host antiviral immunity that can effectively limit disease potential. We used a nonhuman primate model of CMV infection to assess the relationship between CMV disease and the levels of developing anti-CMV immunity. Naive rhesus macaques were inoculated with rhesus cytomegalovirus (RhCMV) followed 2 or 11 weeks later by inoculation with pathogenic simian immunodeficiency virus SIVmac239. Two of four monkeys inoculated with SIV at 2 weeks after inoculation with RhCMV died within 11 weeks with simian AIDS (SAIDS), including activated RhCMV infection. Neither animal had detectable anti-SIV antibodies. The other two animals died 17 and 27 weeks after SIV inoculation with either SAIDS or early lymphoid depletion, although no histological evidence of activated RhCMV was observed. Both had weak anti-SIV antibody titers. RhCMV antibody responses for this group of monkeys were significantly below those of control animals inoculated with only RhCMV. In addition, all animals of this group had persistent RhCMV DNA in plasma and high copy numbers of RhCMV in tissues. In contrast, animals that were inoculated with SIV at 11 weeks after RhCMV infection rarely exhibited RhCMV DNA in plasma, had low copy numbers of RhCMV DNA in most tissues, and did not develop early onset of SAIDS or activated RhCMV. SIV antibody titers were mostly robust and sustained in these monkeys. SIV inoculation blunted further development of RhCMV humoral responses, unlike the normal pattern of development in control monkeys following RhCMV inoculation. Anti-RhCMV immunoglobulin G levels and avidity were slightly below control values, but levels maintained were higher than those observed following SIV infection at 2 weeks after RhCMV inoculation. These findings demonstrate that SIV produces long-lasting insults to the humoral immune system beginning very early after SIV infection. The results also indicate that anti-RhCMV immune development at 11 weeks after infection was sufficient to protect the host from acute RhCMV sequelae following SIV infection, in contrast to the lack of protection afforded by only 2 weeks of immune response to RhCMV. As previously observed, monkeys that were not able to mount a significant immune response to SIV were the most susceptible to SAIDS, including activated RhCMV infection. Rapid development of SAIDS in animals inoculated with SIV 2 weeks after RhCMV inoculation suggests that RhCMV can augment SIV pathogenesis, particularly during primary infection by both viruses.
Figures
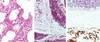
Similar articles
-
Rhesus Cytomegalovirus-Specific CD8+ Cytotoxic T Lymphocytes Do Not Become Functionally Exhausted in Chronic SIVmac239 Infection.Front Immunol. 2020 Aug 12;11:1960. doi: 10.3389/fimmu.2020.01960. eCollection 2020. Front Immunol. 2020. PMID: 32922404 Free PMC article.
-
Cytomegalovirus and simian immunodeficiency virus coinfection: longitudinal study of antibody responses and disease progression.J Acquir Immune Defic Syndr Hum Retrovirol. 1997 May 1;15(1):5-15. doi: 10.1097/00042560-199705010-00002. J Acquir Immune Defic Syndr Hum Retrovirol. 1997. PMID: 9215648
-
Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine.Nature. 2011 May 26;473(7348):523-7. doi: 10.1038/nature10003. Epub 2011 May 11. Nature. 2011. PMID: 21562493 Free PMC article.
-
Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus.Adv Virus Res. 2008;72:207-26. doi: 10.1016/S0065-3527(08)00405-3. Adv Virus Res. 2008. PMID: 19081492 Review.
-
Multi-step pathogenesis of AIDS--role of cytomegalovirus.Res Immunol. 1991 Feb;142(2):87-95. doi: 10.1016/0923-2494(91)90016-c. Res Immunol. 1991. PMID: 1650955 Review.
Cited by
-
Relationship of maternal cytomegalovirus-specific antibody responses and viral load to vertical transmission risk following primary maternal infection in a rhesus macaque model.PLoS Pathog. 2023 Oct 23;19(10):e1011378. doi: 10.1371/journal.ppat.1011378. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37871009 Free PMC article.
-
Horizontal transmission of endemic viruses among rhesus macaques (Macaca mulatta): Implications for human cytomegalovirus vaccine/challenge design.J Med Primatol. 2023 Feb;52(1):53-63. doi: 10.1111/jmp.12621. Epub 2022 Sep 23. J Med Primatol. 2023. PMID: 36151734 Free PMC article.
-
Pathogenesis of Wild-Type-Like Rhesus Cytomegalovirus Strains following Oral Exposure of Immune-Competent Rhesus Macaques.J Virol. 2022 Feb 9;96(3):e0165321. doi: 10.1128/JVI.01653-21. Epub 2021 Nov 17. J Virol. 2022. PMID: 34788083 Free PMC article.
-
Recent Approaches and Strategies in the Generation of Anti-human Cytomegalovirus Vaccines.Methods Mol Biol. 2021;2244:403-463. doi: 10.1007/978-1-0716-1111-1_19. Methods Mol Biol. 2021. PMID: 33555597
-
Maternal Immunity and the Natural History of Congenital Human Cytomegalovirus Infection.Viruses. 2018 Aug 3;10(8):405. doi: 10.3390/v10080405. Viruses. 2018. PMID: 30081449 Free PMC article. Review.
References
-
- Alford, C. A., and W. J. Britt. 1993. Cytomegalovirus, p. 227-255. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, Ltd., New York, N.Y.
-
- Boppana, S. B., and W. J. Britt. 1995. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J. Infect. Dis. 171:1115-1121. - PubMed
-
- Boppana, S. B., K. B. Fowler, W. J. Britt, S. Stagno, and R. F. Pass. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55-60. - PubMed
-
- Boppana, S. B., R. F. Pass, and W. J. Britt. 1993. Virus-specific antibody responses in mothers and their newborn infants with asymptomatic congenital cytomegalovirus infections. J. Infect. Dis. 167:72-77. - PubMed
-
- Bowen, E. F., P. D. Griffiths, C. C. Davey, V. C. Emery, and M. A. Johnson. 1996. Lessons from the natural history of cytomegalovirus. AIDS 10:S37-S41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

