Involvement of the mitogen-activated protein kinase SIMK in regulation of root hair tip growth
- PMID: 12093731
- PMCID: PMC126098
- DOI: 10.1093/emboj/cdf349
Involvement of the mitogen-activated protein kinase SIMK in regulation of root hair tip growth
Abstract
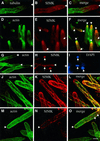
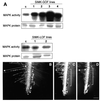
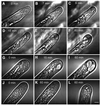
Mitogen-activated protein kinases (MAPKs) are involved in stress signaling to the actin cytoskeleton in yeast and animals. We have analyzed the function of the stress-activated alfalfa MAP kinase SIMK in root hairs. In epidermal cells, SIMK is predominantly nuclear. During root hair formation, SIMK was activated and redistributed from the nucleus into growing tips of root hairs possessing dense F-actin meshworks. Actin depolymerization by latrunculin B resulted in SIMK relocation to the nucleus. Conversely, upon actin stabilization with jasplakinolide, SIMK co-localized with thick actin cables in the cytoplasm. Importantly, latrunculin B and jasplakinolide were both found to activate SIMK in a root-derived cell culture. Loss of tip-focused SIMK and actin was induced by the MAPK kinase inhibitor UO 126 and resulted in aberrant root hairs. UO 126 inhibited targeted vesicle trafficking and polarized growth of root hairs. In contrast, overexpression of gain-of-function SIMK induced rapid tip growth of root hairs and could bypass growth inhibition by UO 126. These data indicate that SIMK plays a crucial role in root hair tip growth.
Figures




Similar articles
-
Involvement of MAP kinase SIMK and actin cytoskeleton in the regulation of root hair tip growth.Cell Biol Int. 2003;27(3):257-9. doi: 10.1016/s1065-6995(02)00344-x. Cell Biol Int. 2003. PMID: 12681328 No abstract available.
-
Convergence and divergence of stress-induced mitogen-activated protein kinase signaling pathways at the level of two distinct mitogen-activated protein kinase kinases.Plant Cell. 2002 Mar;14(3):703-11. Plant Cell. 2002. PMID: 11910015 Free PMC article.
-
SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK.Plant Cell. 2000 Nov;12(11):2247-58. doi: 10.1105/tpc.12.11.2247. Plant Cell. 2000. PMID: 11090222 Free PMC article.
-
The actin cytoskeleton in root hairs: all is fine at the tip.Curr Opin Plant Biol. 2013 Dec;16(6):749-56. doi: 10.1016/j.pbi.2013.10.003. Curr Opin Plant Biol. 2013. PMID: 24446547 Review.
-
Force fluctuation-induced relengthening of acetylcholine-contracted airway smooth muscle.Proc Am Thorac Soc. 2008 Jan 1;5(1):68-72. doi: 10.1513/pats.200705-058VS. Proc Am Thorac Soc. 2008. PMID: 18094087 Free PMC article. Review.
Cited by
-
NQK1/NtMEK1 is a MAPKK that acts in the NPK1 MAPKKK-mediated MAPK cascade and is required for plant cytokinesis.Genes Dev. 2003 Apr 15;17(8):1055-67. doi: 10.1101/gad.1071103. Genes Dev. 2003. PMID: 12704083 Free PMC article.
-
Cellular Complexity in MAPK Signaling in Plants: Questions and Emerging Tools to Answer Them.Front Plant Sci. 2018 Nov 27;9:1674. doi: 10.3389/fpls.2018.01674. eCollection 2018. Front Plant Sci. 2018. PMID: 30538711 Free PMC article. Review.
-
Endocytosis, actin cytoskeleton, and signaling.Plant Physiol. 2004 Jul;135(3):1150-61. doi: 10.1104/pp.104.040683. Plant Physiol. 2004. PMID: 15266049 Free PMC article. Review. No abstract available.
-
Nuclear Signaling of Plant MAPKs.Front Plant Sci. 2018 Apr 11;9:469. doi: 10.3389/fpls.2018.00469. eCollection 2018. Front Plant Sci. 2018. PMID: 29696029 Free PMC article. Review.
-
Self-incompatibility in Papaver: A MAP kinase signals to trigger programmed cell death.Plant Signal Behav. 2008 Apr;3(4):243-5. doi: 10.4161/psb.3.4.5152. Plant Signal Behav. 2008. PMID: 19704642 Free PMC article.
References
-
- Baier R., Schiene,K., Kohring,B., Flaschel,E. and Niehaus,K. (1999) Alfalfa and tobacco cells react differently to chitin oligosaccharides and Sinorhizobium meliloti nodulation factors. Planta, 210, 157–164. - PubMed
-
- Baluška F. and Volkmann,D. (2002) Actin-driven polar growth of plant cells. Trends Cell Biol., 12, 14. - PubMed
-
- Baluška F., Salaj,J., Mathur,J., Braun,M., Jasper,F., Šamaj,J., Chua, N.-H., Barlow,P.W. and Volkmann,D. (2000a) Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol., 227, 618–632. - PubMed
-
- Baluška F., Ovecka,M. and Hirt,H. (2000b) Salt stress- and cell cycle phase-dependent changes in expression and subcellular localisation of the alfalfa mitogen-activated protein kinase SIMK. Protoplasma, 212, 262–267.
-
- Benndorf R., Hayess,K., Ryazantsev,S., Wieske,M., Behlke,J. and Lutsch,G. (1994) Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J. Biol. Chem., 269, 20780–20784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

