Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis
- PMID: 12082083
- PMCID: PMC2173539
- DOI: 10.1083/jcb.200202067
Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis
Abstract
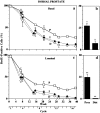
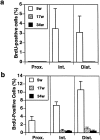
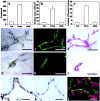
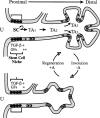
Stem cells are believed to regulate normal prostatic homeostasis and to play a role in the etiology of prostate cancer and benign prostatic hyperplasia. We show here that the proximal region of mouse prostatic ducts is enriched in a subpopulation of epithelial cells that exhibit three important attributes of epithelial stem cells: they are slow cycling, possess a high in vitro proliferative potential, and can reconstitute highly branched glandular ductal structures in collagen gels. We propose a model of prostatic homeostasis in which mouse prostatic epithelial stem cells are concentrated in the proximal region of prostatic ducts while the transit-amplifying cells occupy the distal region of the ducts. This model can account for many biological differences between cells of the proximal and distal regions, and has implications for prostatic disease formation.
Figures



Similar articles
-
Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue.Proc Natl Acad Sci U S A. 2005 May 17;102(20):7180-5. doi: 10.1073/pnas.0502761102. Proc Natl Acad Sci U S A. 2005. PMID: 15899981 Free PMC article.
-
Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis.Stem Cells. 2006 Aug;24(8):1859-68. doi: 10.1634/stemcells.2005-0585. Epub 2006 Apr 27. Stem Cells. 2006. PMID: 16644920
-
TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts.J Cell Biol. 2005 Jul 4;170(1):81-90. doi: 10.1083/jcb.200412015. Epub 2005 Jun 27. J Cell Biol. 2005. PMID: 15983059 Free PMC article.
-
Epithelial stem cells in human prostate growth and disease.Prostate Cancer Prostatic Dis. 2004;7(3):188-94. doi: 10.1038/sj.pcan.4500745. Prostate Cancer Prostatic Dis. 2004. PMID: 15289813 Review.
-
Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model.Prostate. 1996 Feb;28(2):98-106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. Prostate. 1996. PMID: 8604398 Review.
Cited by
-
Identification of CD166 as a surface marker for enriching prostate stem/progenitor and cancer initiating cells.PLoS One. 2012;7(8):e42564. doi: 10.1371/journal.pone.0042564. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22880034 Free PMC article.
-
Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats.J Clin Invest. 2012 Apr;122(4):1567-73. doi: 10.1172/JCI58789. Epub 2012 Mar 12. J Clin Invest. 2012. PMID: 22406533 Free PMC article.
-
Meta-analyses of mouse and human prostate single-cell transcriptomes reveal widespread epithelial plasticity in tissue regression, regeneration, and cancer.bioRxiv [Preprint]. 2024 Feb 2:2024.01.30.578066. doi: 10.1101/2024.01.30.578066. bioRxiv. 2024. Update in: Genome Med. 2025 Jan 17;17(1):5. doi: 10.1186/s13073-025-01432-w. PMID: 38352515 Free PMC article. Updated. Preprint.
-
A luminal epithelial stem cell that is a cell of origin for prostate cancer.Nature. 2009 Sep 24;461(7263):495-500. doi: 10.1038/nature08361. Epub 2009 Sep 9. Nature. 2009. PMID: 19741607 Free PMC article.
-
Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications.Medicines (Basel). 2019 Jul 30;6(3):82. doi: 10.3390/medicines6030082. Medicines (Basel). 2019. PMID: 31366128 Free PMC article. Review.
References
-
- Baldwin, B.H., B.C. Tennant, T.J. Reimers, R.G. Cowan, and P.W. Concannon. 1985. Circannual changes in serum testosterone concentrations of adult and yearling woodchucks (Marmota monax). Biol. Reprod. 32:804–812. - PubMed
-
- Banerjee, P.P., S. Banerjee, B.R. Zirkin, and T.R. Brown. 1998. Telomerase activity in normal adult Brown Norway rat seminal vesicle: regional distribution and age-dependent changes. Endocrinology. 139:1075–1081. - PubMed
-
- Berardi, A.C., A. Wang, J.D. Levine, P. Lopez, and D.T. Scadden. 1995. Functional isolation and characterization of human hematopoietic stem cells. Science. 267:104–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

