Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239
- PMID: 12072518
- PMCID: PMC136301
- DOI: 10.1128/jvi.76.14.7187-7202.2002
Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239
Abstract
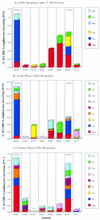
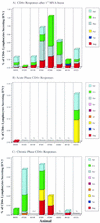
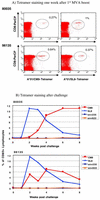
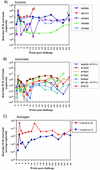
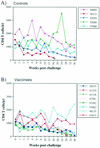
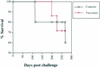
Producing a prophylactic vaccine for human immunodeficiency virus (HIV) has proven to be a challenge. Most biological isolates of HIV are difficult to neutralize, so that conventional subunit-based antibody-inducing vaccines are unlikely to be very effective. In the rhesus macaque model, some protection was afforded by DNA/recombinant viral vector vaccines. However, these studies used as the challenge virus SHIV-89.6P, which is neutralizable, making it difficult to determine whether the observed protection was due to cellular immunity, humoral immunity, or a combination of both. In this study, we used a DNA prime/modified vaccinia virus Ankara boost regimen to immunize rhesus macaques against nearly all simian immunodeficiency virus (SIV) proteins. These animals were challenged intrarectally with pathogenic molecularly cloned SIVmac239, which is resistant to neutralization. The immunization regimen resulted in the induction of virus-specific CD8(+) and CD4(+) responses in all vaccinees. Although anamnestic neutralizing antibody responses against laboratory-adapted SIVmac251 developed after the challenge, no neutralizing antibodies against SIVmac239 were detectable. Vaccinated animals had significantly reduced peak viremia compared with controls (P < 0.01). However, despite the induction of virus-specific cellular immune responses and reduced peak viral loads, most animals still suffered from gradual CD4 depletion and progressed to disease.
Figures

Similar articles
-
Reduction of viral loads by multigenic DNA priming and adenovirus boosting in the SIVmac-macaque model.Vaccine. 2006 Mar 10;24(11):1811-20. doi: 10.1016/j.vaccine.2005.10.026. Epub 2005 Oct 25. Vaccine. 2006. PMID: 16274888
-
Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239.J Virol. 2003 Dec;77(24):13348-60. doi: 10.1128/jvi.77.24.13348-13360.2003. J Virol. 2003. PMID: 14645590 Free PMC article.
-
CD8 T Cells Show Protection against Highly Pathogenic Simian Immunodeficiency Virus (SIV) after Vaccination with SIV Gene-Expressing BCG Prime and Vaccinia Virus/Sendai Virus Vector Boosts.J Virol. 2021 Jan 28;95(4):e01718-20. doi: 10.1128/JVI.01718-20. Print 2021 Jan 28. J Virol. 2021. PMID: 33087465 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine.Vaccine. 2002 May 6;20(15):1949-55. doi: 10.1016/s0264-410x(02)00076-2. Vaccine. 2002. PMID: 11983252 Review.
Cited by
-
Heat-inactivated modified vaccinia virus Ankara boosts Th1 cellular and humoral immunity as a vaccine adjuvant.NPJ Vaccines. 2022 Oct 19;7(1):120. doi: 10.1038/s41541-022-00542-5. NPJ Vaccines. 2022. PMID: 36261460 Free PMC article.
-
Inclusion of a CRF01_AE HIV envelope protein boost with a DNA/MVA prime-boost vaccine: Impact on humoral and cellular immunogenicity and viral load reduction after SHIV-E challenge.Vaccine. 2012 Feb 27;30(10):1830-40. doi: 10.1016/j.vaccine.2011.12.131. Epub 2012 Jan 9. Vaccine. 2012. PMID: 22234262 Free PMC article.
-
In vivo CD8+ T cell control of immunodeficiency virus infection in humans and macaques.Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6365-70. doi: 10.1073/pnas.0700666104. Epub 2007 Apr 2. Proc Natl Acad Sci U S A. 2007. PMID: 17404226 Free PMC article.
-
Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses.J Virol. 2003 Mar;77(5):3099-118. doi: 10.1128/jvi.77.5.3099-3118.2003. J Virol. 2003. PMID: 12584336 Free PMC article.
-
The hope for an HIV vaccine based on induction of CD8+ T lymphocytes--a review.Mem Inst Oswaldo Cruz. 2008 Mar;103(2):119-29. doi: 10.1590/s0074-02762008000200001. Mem Inst Oswaldo Cruz. 2008. PMID: 18425263 Free PMC article. Review.
References
-
- Ahmad, S., B. Lohman, M. Marthas, L. Giavedoni, Z. el-Amad, N. L. Haigwood, C. J. Scandella, M. B. Gardner, P. A. Luciw, and T. Yilma. 1994. Reduced virus load in rhesus macaques immunized with recombinant gp160 and challenged with simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 10:195-204. - PubMed
-
- Allen, T. M., B. R. Mothe, J. Sidney, P. Jing, J. L. Dzuris, M. E. Liebl, T. U. Vogel, D. H. O'Connor, X. Wang, M. C. Wussow, J. A. Thomson, J. D. Altman, D. I. Watkins, and A. Sette. 2001. CD8+ lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule mamu-A*01: implications for vaccine design and testing. J. Virol. 75:738-749. - PMC - PubMed
-
- Allen, T. M., D. H. O'Connor, J. Peicheng, J. L. Dzuris, B. R. Mothé, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viremia. Nature 407:386-390. - PubMed
-
- Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. - PubMed
-
- Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

