GATA-2 and GATA-2/ER display opposing activities in the development and differentiation of blood progenitors
- PMID: 12065419
- PMCID: PMC126056
- DOI: 10.1093/emboj/cdf301
GATA-2 and GATA-2/ER display opposing activities in the development and differentiation of blood progenitors
Abstract
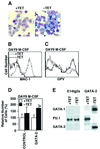
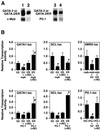
GATA-2 is a zinc finger transcription factor essential for the development of hematopoiesis. While GATA-2 is generally considered to play an important role in the biology of hematopoietic stem and progenitor cells, its function within these compartments is not well understood. Here we have employed both conditional expression of GATA-2 and conditional activation of a GATA-2/estrogen receptor (ER) chimera to examine the effect of enforced GATA-2 expression in the development and differentiation of hematopoietic progenitors from murine embryonic stem cells. Consistent with the phenotype of GATA-2 null animals, conditional expression of GATA-2 from a tetracycline-inducible promoter enhanced the production of hematopoietic progenitors. Conditional activation of a GATA-2/ER chimera produced essentially opposite effects to those observed with conditional GATA-2 expression. GATA-2 and GATA-2/ER differ in their binding activities and transcriptional interactions from other hematopoietic-associated transcription factors such as c-Myb and PU.1. While we have exploited these differences in activity to explore the transcriptional networks underlying hematopoietic cell fate determination, our results suggest that care should be taken in interpreting results obtained using only chimeric proteins.
Figures

Similar articles
-
RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells.Development. 2005 Mar;132(5):1117-26. doi: 10.1242/dev.01660. Epub 2005 Feb 2. Development. 2005. PMID: 15689374
-
Expression and genetic interaction of transcription factors GATA-2 and GATA-3 during development of the mouse central nervous system.Dev Biol. 1999 Jun 15;210(2):305-21. doi: 10.1006/dbio.1999.9278. Dev Biol. 1999. PMID: 10357893
-
Essential role of Gata transcription factors in sympathetic neuron development.Development. 2004 Oct;131(19):4775-86. doi: 10.1242/dev.01370. Epub 2004 Aug 25. Development. 2004. PMID: 15329349
-
Adrenocortical tumorigenesis in transgenic mice: the role of luteinizing hormone receptor and transcription factors GATA-4 and GATA-61.Reprod Biol. 2001 Jul;1(1):5-9. Reprod Biol. 2001. PMID: 14666170 Review.
-
Transcriptional regulation of erythropoiesis: an affair involving multiple partners.Oncogene. 2002 May 13;21(21):3368-76. doi: 10.1038/sj.onc.1205326. Oncogene. 2002. PMID: 12032775 Review.
Cited by
-
Conditional overexpression of transgenes in megakaryocytes and platelets in vivo.Blood. 2005 Sep 1;106(5):1559-64. doi: 10.1182/blood-2005-02-0638. Epub 2005 May 12. Blood. 2005. PMID: 15890684 Free PMC article.
-
Multipotential differentiation ability of GATA-1-null erythroid-committed cells.Genes Dev. 2006 Mar 15;20(6):654-9. doi: 10.1101/gad.1378206. Genes Dev. 2006. PMID: 16543218 Free PMC article.
-
Vasculogenic and hematopoietic cellular progenitors are scattered within the prenatal mouse heart.Histochem Cell Biol. 2015 Feb;143(2):153-69. doi: 10.1007/s00418-014-1269-z. Epub 2014 Sep 9. Histochem Cell Biol. 2015. PMID: 25201347 Free PMC article.
-
GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling.Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8811-6. doi: 10.1073/pnas.1432147100. Epub 2003 Jul 11. Proc Natl Acad Sci U S A. 2003. PMID: 12857954 Free PMC article.
-
Regulation of the MDM2-P53 pathway and tumor growth by PICT1 via nucleolar RPL11.Nat Med. 2011 Jul 31;17(8):944-51. doi: 10.1038/nm.2392. Nat Med. 2011. PMID: 21804542 Free PMC article.
References
-
- Briegel K., Lim,K.C., Plank,C., Beug,H., Engel,J.D. and Zenke,M. (1993) Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev., 7, 1097–1109. - PubMed
-
- Era T., Takagi,T., Takahashi,T., Bories,J.C. and Nakano,T. (2000) Characterization of hematopoietic lineage-specific gene expression by ES cell in vitro differentiation induction system. Blood, 95, 870–878. - PubMed
-
- Evans T. and Felsenfeld,G. (1989) The erythroid-specific transcription factor Eryf1: a new finger protein. Cell, 58, 877–885. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

