ADP ribosylation of human neutrophil peptide-1 regulates its biological properties
- PMID: 12060767
- PMCID: PMC123050
- DOI: 10.1073/pnas.122238899
ADP ribosylation of human neutrophil peptide-1 regulates its biological properties
Abstract
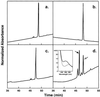
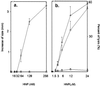
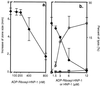
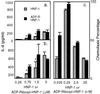
In human airways, epithelial cells lining the lumen and intraluminal cells (e.g., polymorphonuclear cells) participate in the innate immune response. These cells secrete or express on their surfaces arginine-specific ADP ribosyltransferases. Defensins, antimicrobial proteins secreted by immune cells, are arginine-rich, leading us to hypothesize that ADP ribosylation could modify their biological activities. We found that an arginine-specific ADP ribosyltransferase-1 present on airway epithelial cells modifies Arg-14 of alpha defensin-1. ADP-ribosylated defensin-1 had decreased antimicrobial and cytotoxic activities but still stimulated T cell chemotaxis and IL-8 release from A549 cells. Further, ADP-ribosylated defensin-1 inhibited cytotoxic and antimicrobial activities of unmodified defensin-1. We identified ADP-ribosylated defensin-1 in bronchoalveolar lavage fluid from smokers but not from nonsmokers, confirming its existence in vivo. Thus, airway mono-ADP-ribosyltransferases could have an important regulatory role in the innate immune response through modification of alpha defensin-1 and perhaps other basic molecules, with alteration of their biological properties.
Figures
Similar articles
-
Mono-ADP-ribosylation: a tool for modulating immune response and cell signaling.Sci STKE. 2002 Dec 17;2002(163):pe53. doi: 10.1126/stke.2002.163.pe53. Sci STKE. 2002. PMID: 12488509 Review.
-
ADP-ribosylation of human defensin HNP-1 results in the replacement of the modified arginine with the noncoded amino acid ornithine.Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):19796-800. doi: 10.1073/pnas.0910633106. Epub 2009 Nov 6. Proc Natl Acad Sci U S A. 2009. PMID: 19897717 Free PMC article.
-
ADP-ribosyltransferase-specific modification of human neutrophil peptide-1.J Biol Chem. 2006 Jun 23;281(25):17054-17060. doi: 10.1074/jbc.M603042200. Epub 2006 Apr 20. J Biol Chem. 2006. PMID: 16627471
-
Specificity and target proteins of arginine-specific mono-ADP-ribosylation in T-tubules of rabbit skeletal muscle.Arch Biochem Biophys. 1997 Nov 15;347(2):155-62. doi: 10.1006/abbi.1997.0330. Arch Biochem Biophys. 1997. PMID: 9367520
-
Characterization of glycosylphosphatidylinositiol-anchored, secreted, and intracellular vertebrate mono-ADP-ribosyltransferases.Annu Rev Nutr. 1999;19:485-509. doi: 10.1146/annurev.nutr.19.1.485. Annu Rev Nutr. 1999. PMID: 10448534 Review.
Cited by
-
Arginine-specific mono ADP-ribosylation in vitro of antimicrobial peptides by ADP-ribosylating toxins.PLoS One. 2012;7(8):e41417. doi: 10.1371/journal.pone.0041417. Epub 2012 Aug 7. PLoS One. 2012. PMID: 22879887 Free PMC article.
-
Extracellular NAD and ATP: Partners in immune cell modulation.Purinergic Signal. 2007 Mar;3(1-2):71-81. doi: 10.1007/s11302-006-9038-7. Epub 2007 Jan 9. Purinergic Signal. 2007. PMID: 18404420 Free PMC article.
-
Transcriptome sequencing analysis of mono‑ADP‑ribosylation in colorectal cancer cells.Oncol Rep. 2020 May;43(5):1413-1428. doi: 10.3892/or.2020.7516. Epub 2020 Feb 24. Oncol Rep. 2020. PMID: 32323815 Free PMC article.
-
Basal and inducible expression of the thiol-sensitive ART2.1 ecto-ADP-ribosyltransferase in myeloid and lymphoid leukocytes.Purinergic Signal. 2009 Sep;5(3):369-83. doi: 10.1007/s11302-009-9162-2. Epub 2009 Apr 30. Purinergic Signal. 2009. PMID: 19404775 Free PMC article.
-
Dysregulation of human beta-defensin-2 protein in inflammatory bowel disease.PLoS One. 2009 Jul 20;4(7):e6285. doi: 10.1371/journal.pone.0006285. PLoS One. 2009. PMID: 19617917 Free PMC article.
References
-
- Nakamura H, Yoshimura K, Jaffe H A, Crystal R G. J Biol Chem. 1991;266:19611–19617. - PubMed
-
- Rihman-Eisenstat J B, Jorens P G, Herbert C A, Ueki I, Nadel J A. Am J Physiol. 1993;264:L413–L418. - PubMed
-
- Pardi A, Zhang X L, Selsted M E, Skalicky J J, Yip P F. Biochemistry. 1992;31:11357–11364. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

