Dissociation of the tubulin dimer is extremely slow, thermodynamically very unfavorable, and reversible in the absence of an energy source
- PMID: 12058074
- PMCID: PMC117629
- DOI: 10.1091/mbc.e01-10-0089
Dissociation of the tubulin dimer is extremely slow, thermodynamically very unfavorable, and reversible in the absence of an energy source
Abstract
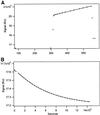
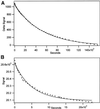
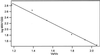
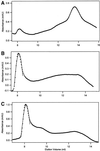
The finding that exchange of tubulin subunits between tubulin dimers (alpha-beta + alpha'beta' <--> alpha'beta + alphabeta') does not occur in the absence of protein cofactors and GTP hydrolysis conflicts with the assumption that pure tubulin dimer and monomer are in rapid equilibrium. This assumption underlies the many physical chemical measurements of the K(d) for dimer dissociation. To resolve this discrepancy we used surface plasmon resonance to determine the rate constant for dimer dissociation. The half-time for dissociation was approximately 9.6 h with tubulin-GTP, 2.4 h with tubulin-GDP, and 1.3 h in the absence of nucleotide. A Kd equal to 10(-11) M was calculated from the measured rate for dissociation and an estimated rate for association. Dimer dissociation was found to be reversible, and dimer formation does not require GTP hydrolysis or folding information from protein cofactors, because 0.2 microM tubulin-GDP incubated for 20 h was eluted as dimer when analyzed by size exclusion chromatography. Because 20 h corresponds to eight half-times for dissociation, only monomer would be present if dissociation were an irreversible reaction and if dimer formation required GTP or protein cofactors. Additional evidence for a 10(-11) M K(d) was obtained from gel exclusion chromatography studies of 0.02-2 nM tubulin-GDP. The slow dissociation of the tubulin dimer suggests that protein tubulin cofactors function to catalyze dimer dissociation, rather than dimer assembly. Assuming N-site-GTP dissociation is from monomer, our results agree with the 16-h half-time for N-site GTP in vitro and 33 h half-life for tubulin N-site-GTP in CHO cells.
Figures

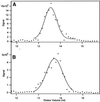
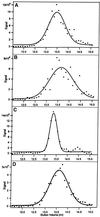
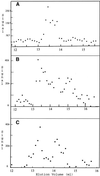

Similar articles
-
Concerning the chemical nature of tubulin subunits that cap and stabilize microtubules.Biochemistry. 2003 Feb 25;42(7):2122-6. doi: 10.1021/bi027010s. Biochemistry. 2003. PMID: 12590601
-
Control of the structural stability of the tubulin dimer by one high affinity bound magnesium ion at nucleotide N-site.J Biol Chem. 1998 Jan 2;273(1):167-76. doi: 10.1074/jbc.273.1.167. J Biol Chem. 1998. PMID: 9417061
-
Equilibrium studies of a fluorescent paclitaxel derivative binding to microtubules.Biochemistry. 2000 Jan 25;39(3):616-23. doi: 10.1021/bi992044u. Biochemistry. 2000. PMID: 10642187
-
Linkages between the dissociation of alpha beta tubulin into subunits and ligand binding: the ground state of tubulin is the GDP conformation.Biochemistry. 1994 Feb 1;33(4):885-93. doi: 10.1021/bi00170a006. Biochemistry. 1994. PMID: 8305436
-
Tubulin folding cofactors: half a dozen for a dimer.Curr Biol. 2002 Nov 19;12(22):R767-9. doi: 10.1016/s0960-9822(02)01288-5. Curr Biol. 2002. PMID: 12445400 Review.
Cited by
-
The rate and equilibrium constants for a multistep reaction sequence for the aggregation of superoxide dismutase in amyotrophic lateral sclerosis.Proc Natl Acad Sci U S A. 2004 Oct 19;101(42):15094-9. doi: 10.1073/pnas.0406650101. Epub 2004 Oct 8. Proc Natl Acad Sci U S A. 2004. PMID: 15475574 Free PMC article.
-
Bacterial tubulin distinct loop sequences and primitive assembly properties support its origin from a eukaryotic tubulin ancestor.J Biol Chem. 2011 Jun 3;286(22):19789-803. doi: 10.1074/jbc.M111.230094. Epub 2011 Apr 4. J Biol Chem. 2011. PMID: 21467045 Free PMC article.
-
Tubulin binds to the cytoplasmic loop of TRESK background K⁺ channel in vitro.PLoS One. 2014 May 15;9(5):e97854. doi: 10.1371/journal.pone.0097854. eCollection 2014. PLoS One. 2014. PMID: 24830385 Free PMC article.
-
Revisiting the tubulin cofactors and Arl2 in the regulation of soluble αβ-tubulin pools and their effect on microtubule dynamics.Mol Biol Cell. 2017 Feb 1;28(3):359-363. doi: 10.1091/mbc.E15-10-0694. Mol Biol Cell. 2017. PMID: 28137948 Free PMC article.
-
Tubulin heterodimers remain functional for one cell cycle after the inactivation of tubulin-folding cofactor D in fission yeast cells.Yeast. 2009 Apr;26(4):235-47. doi: 10.1002/yea.1663. Yeast. 2009. PMID: 19330768 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

